Method for preparing acacetin by enzymatic hydrolysis of buddleoside
A technology for preparing gold and acacetin from anthocyanin, which is applied in the field of bioengineering, can solve the problems such as the lack of examples of enzymatic hydrolysis of anthocyanin, and achieve the effects of high product purity, strong reaction specificity, and reduced separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
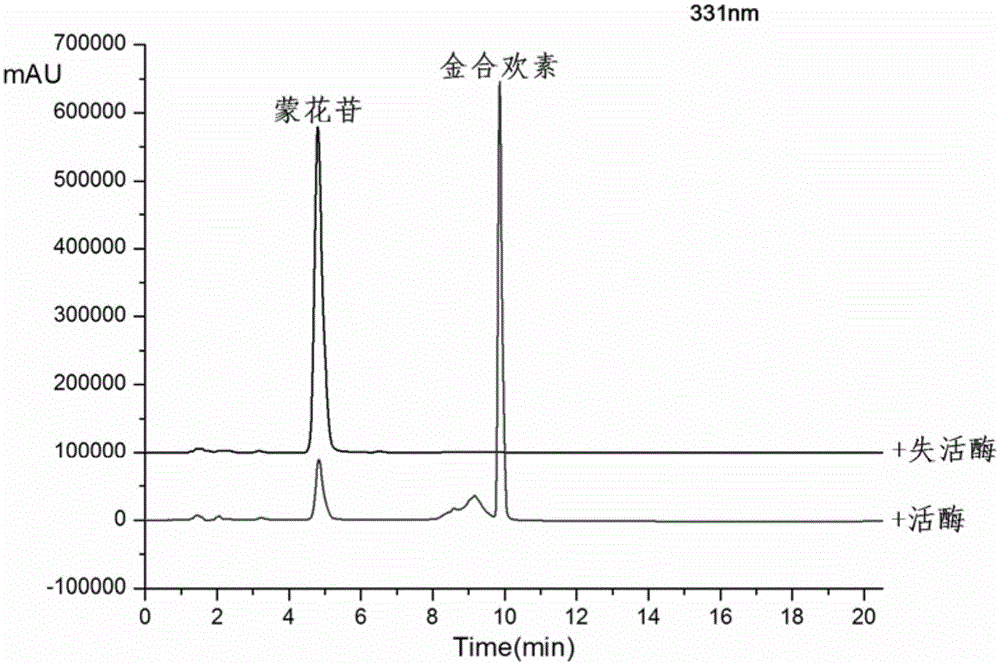
[0023] Take a 100ml Erlenmeyer flask and add 38.5ml Tris-HCl buffer (pH7.6), 7.5ml crude enzyme solution, 3.6ml HP-β-CD solution, 1.25ml 20mM mongoside solution, 0.4ml 1M CaCl2 solution, after closing the bottle mouth, reacted at 200rpm rotary shaker at 35°C for 1h, and carried out HPLC analysis after the reaction (detection wavelength 330nm, mobile phase: acetonitrile (B) / pure water (A), gradient is, 0.0-4.0min, 30%-42%B; 4.0-4.5min, 42%-70%B; 4.5-12min, 70%-90%B; 12-16min, 90%-90%B; 16.0-16.5, 90%-30% B). Such as figure 1 It was shown that after 1 hour of reaction, the conversion rate of mongoside was above 85%, and the product was acacetin. It can be seen that the enzyme can efficiently convert mongoside.
Embodiment 2
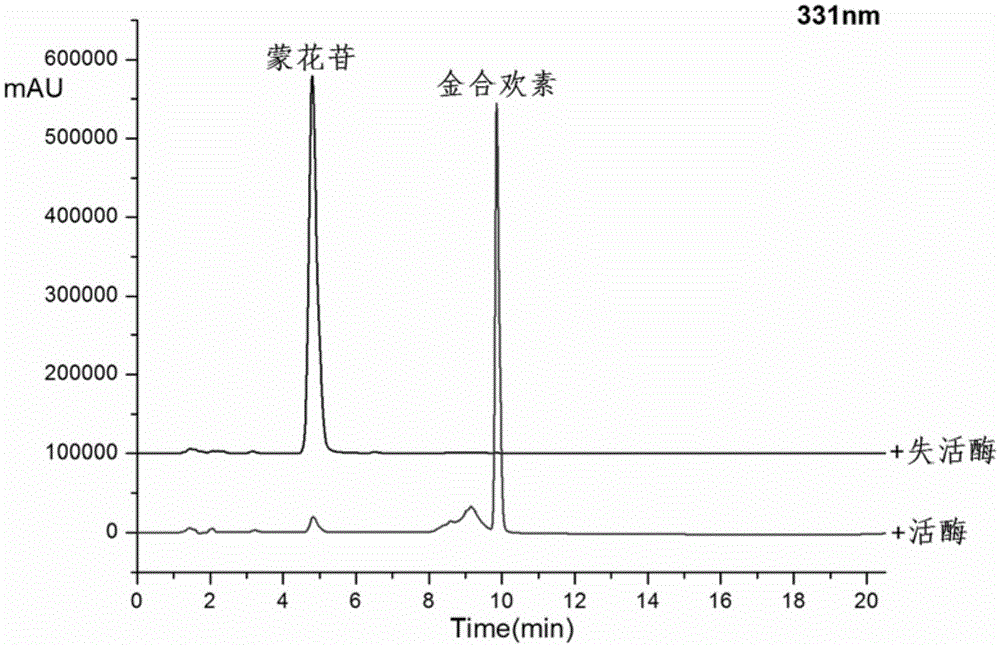
[0025] Take a 100ml Erlenmeyer flask and add 41ml Tris-HCl buffer solution (pH7.6), 5ml crude enzyme solution, 3.6ml HP-β-CD solution, 1.25ml concentration of 20mM mongoside solution, 0.4ml concentration of 1M CaCl2 Solution, after closing the bottle mouth, reacted at 200rpm rotary shaker at 35°C for 2h, and carried out HPLC analysis after the reaction (detection wavelength 330nm, mobile phase: acetonitrile (B) / pure water (A), gradient: 0.0- 4.0min, 30%-42%B; 4.0-4.5min, 42%-70%B; 4.5-12min, 70%-90%B; 12-16min, 90%-90%B; 16.0-16.5, 90% -30% B). Such as figure 2 As shown, after 2 hours of reaction, the conversion rate of mongoside is above 96%, and the product is acacetin, which shows the potential advantage of the enzyme in efficiently preparing acacetin.
Embodiment 3
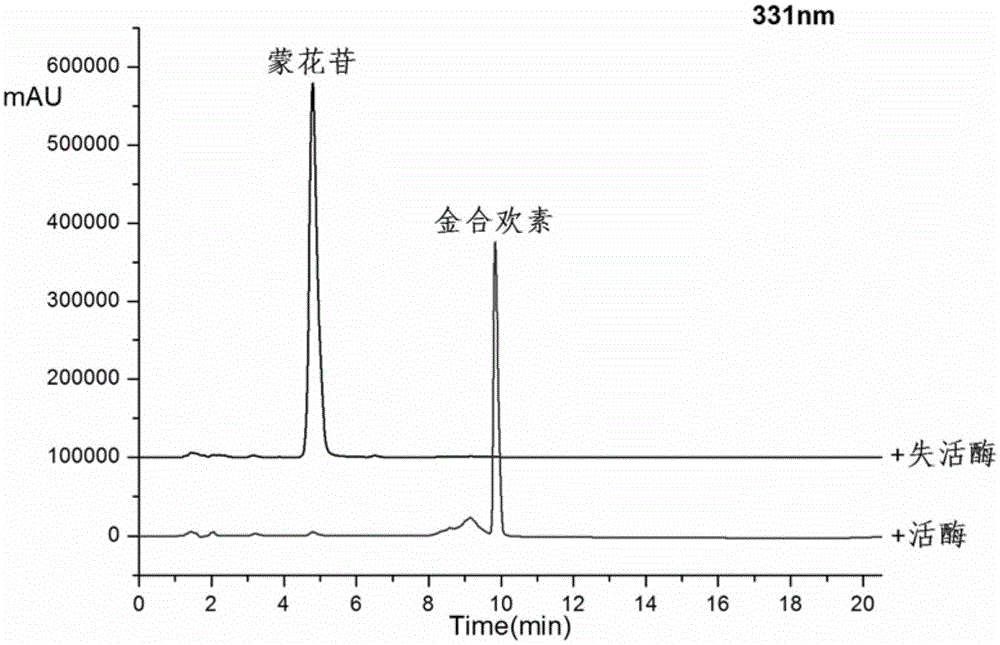
[0027] Take a 100ml Erlenmeyer flask and add 43.5ml Tris-HCl buffer (pH7.6), 2.5ml crude enzyme solution, 3.6ml HP-β-CD solution, 1.25ml 20mM mongoside solution, 0.4ml 1M CaCl2 solution, after closing the bottle mouth, reacted at 200rpm rotary shaker at 35°C for 6h, and carried out HPLC analysis after the reaction (detection wavelength 330nm, mobile phase: acetonitrile (B) / pure water (A), gradient is, 0.0-3.0min, 30%-42%B; 4.0-4.5min, 42%-70%B; 4.5-12min, 70%-90%B; 12-16min, 90%-90%B; 16.0-16.5, 90%-30% B). Such as image 3 It can be seen that after 6 hours of reaction, mongoside is almost completely converted, and the product is acacetin. It can be seen that the crude enzyme solution can be used as a tool for efficiently preparing acacetin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com