Application of rhein or curcumin in preparation of medicines for preventing and/or treating diabetic nephropathy
A technology of diabetic nephropathy and rhein, applied in drug combinations, urinary system diseases, metabolic diseases, etc., can solve the symptoms of metabolic acidosis without improving or protecting kidney function, effectively slowing down the progression of the disease, and aggravating renal failure And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] cell experiment
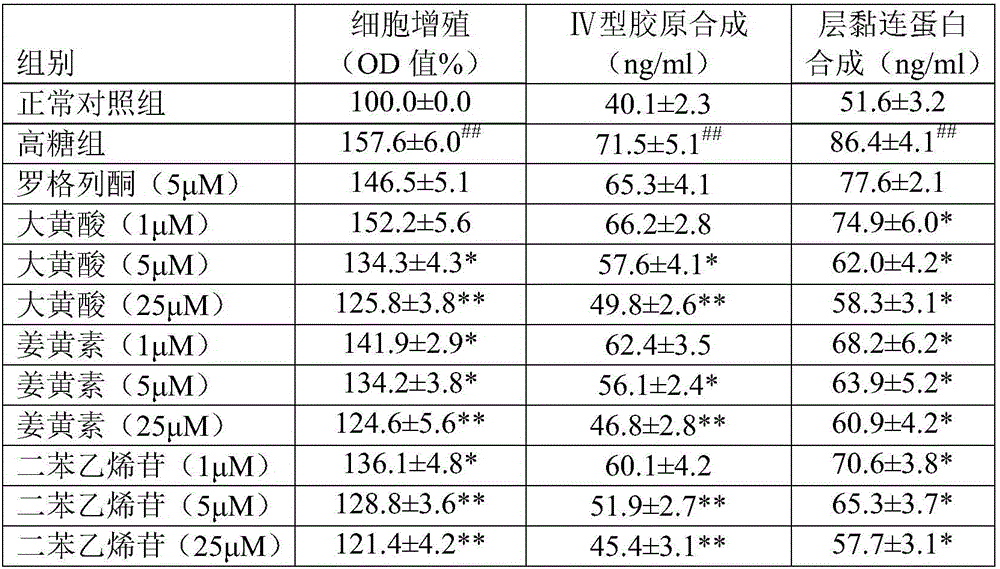
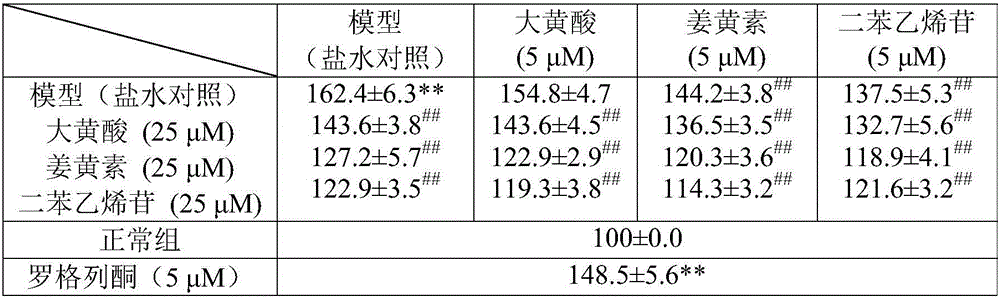
[0028] This embodiment mainly evaluates the effect and dose-effect relationship of each component. Using the glomerular mesangial cells (EMCs) high-glucose culture model, the effects of rhein, curcumin and stilbene glycosides on the proliferation of EMCs and the synthesis of type IV collagen and laminin were screened.
[0029] 1. MTT colorimetric method to measure the proliferation of rat glomerular mesangial cells (MCs)
[0030] MCs significantly stimulated mesangial cell proliferation when cultured in high glucose environment for 48h.
[0031] MCs were cultured in DMEM medium (purchased from gibco) containing 10% fetal bovine serum at 37 °C, 5% CO 2 Incubated in an incubator, digested with 0.25% trypsin and passaged 2-3 times a week. MTT method was used to detect the proliferation activity of GMCs, and the logarithmic phase rat GMCs were taken, and each well was 2×10 4 -4×10 4 The cells were seeded on a 96-well plate, and after 12 hours of subco...
Embodiment 2
[0062] rat experiment
[0063] Streptozotocin (STZ)-induced type II diabetes rat model
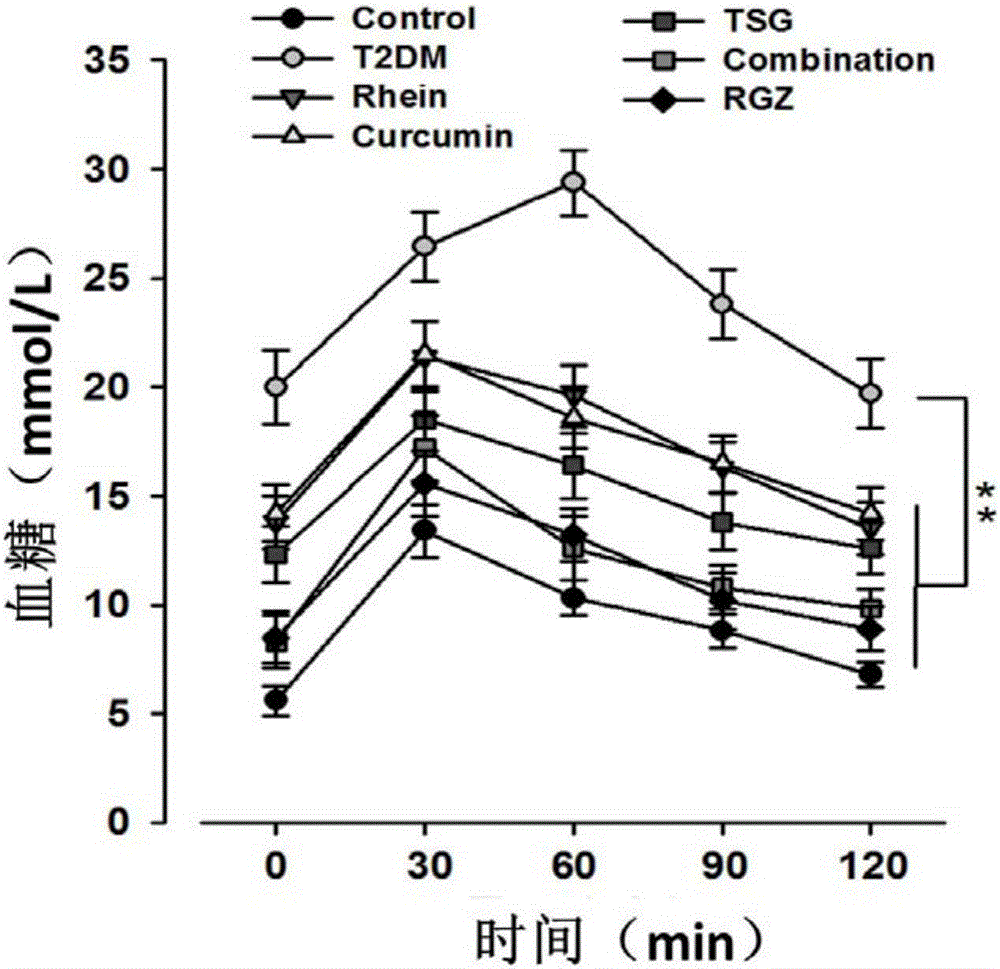
[0064] SD (Spraque-Dawley) male rats, about 10 weeks old, weighing 252±23g, were randomly divided into normal control group and diabetes model group. After 1 week of adaptive feeding, the rats in the model group were fed with high-sugar and high-fat feed (containing 10.0% lard, 20.0% sucrose, 2.5% cholesterol, 1.0% cholate, and 66.5% conventional feed) for 4 weeks to induce Insulin resistance develops. In the fifth week, after the rats in each experimental group were fasted for 12 hours, 30 mg / kg of Streptozotocin (STZ) was intraperitoneally injected, and the same volume of citrate buffer was injected intraperitoneally in the normal control group. One week after the injection, the rats in each group were fasted for 12 hours, then their tails were docked to collect blood, and the fasting blood glucose (FBG) was measured. FBG>16.7mmol / L was considered successful in modeling. The treatmen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com