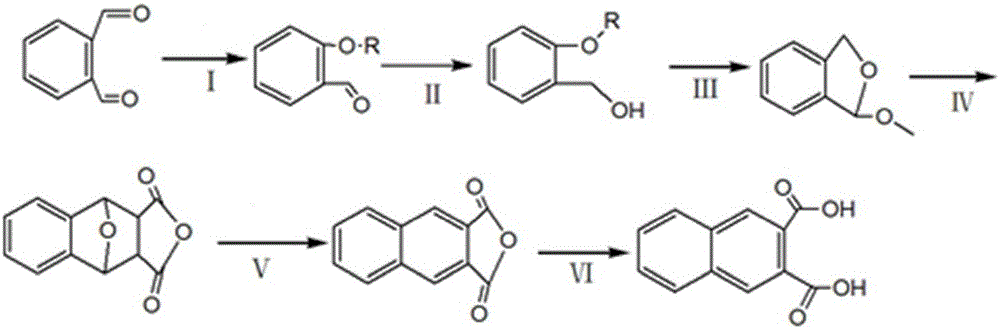
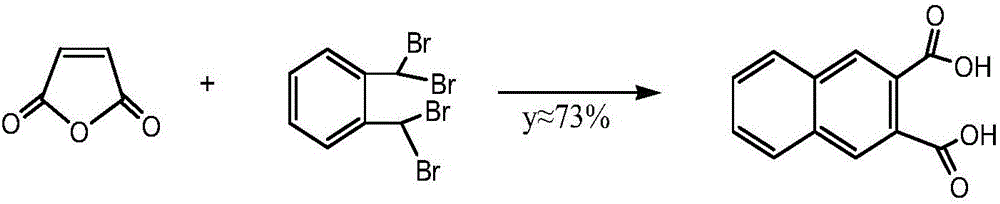Preparation method of 2,3-naphthalic acid
A technology of naphthalene dicarboxylic acid and phthalaldehyde monoacetal, which is applied in the preparation of carboxylate/lactone, organic chemistry, etc. It can solve the problems of difficult industrial production, high environmental pollution, and poor product purity. It is not easy to achieve The effect of obtaining, good purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] A kind of preparation method of 2,3-naphthalene dicarboxylic acid of the present invention, concrete implementation steps are as follows:
[0044] (I) Acetalization reaction: Add the reactant o-phthalaldehyde and alcohols with 2 to 4 carbon atoms, acid catalyst I and cyclohexane into the reaction vessel, and o-phthalaldehyde and alcohols with 2 to 4 carbon atoms The molar ratio of alcohols is 1:1~1.5, the addition amount of acidic catalyst I is 5~8% of the total reactant mass fraction, the addition of cyclohexane is 2~4 times of the total reactant mass, and the reflux fraction Water for 3 to 8 hours to obtain o-phthalaldehyde monoacetal; wherein the alcohols with 2 to 4 carbon atoms are any one of ethylene glycol, propylene glycol, isopropanol, n-butanol or isobutanol; acidic Catalyst I is any one of phosphoric acid, p-toluenesulfonic acid, potassium bisulfate or cation exchange resin.
[0045] (II) Reduction reaction: Heat tetrahydrofuran and reducing agent sodium bor...
Embodiment 1
[0051] (I) Acetalization reaction: Add the reactants o-phthalaldehyde, ethylene glycol, phosphoric acid, and cyclohexane into the reaction vessel, the molar ratio of o-phthalaldehyde and cyclohexane is 1:1, and the acidic catalyst I The added amount is 5% of the mass fraction of the total reactants, the added amount of cyclohexane is 2-4 times of the total reactant mass, heating and reflux for water separation for 3-8 hours to obtain o-phthalaldehyde monoacetal.
[0052] (II) Reduction reaction: After heating tetrahydrofuran and sodium borohydride to reflux for 0.5 hours, slowly add o-phthalaldehyde monoacetal for reduction reaction, the molar ratio of phthalaldehyde monoacetal and tetrahydrofuran is 1:2, reducing agent and The molar ratio of o-phthalaldehyde monoacetal is 1:1, continue to reflux for 1.5 hours after adding, and then add water and hydrochloric acid solution with a concentration of 25% in the micro-reflux state to terminate the reaction, the volume ratio of hydro...
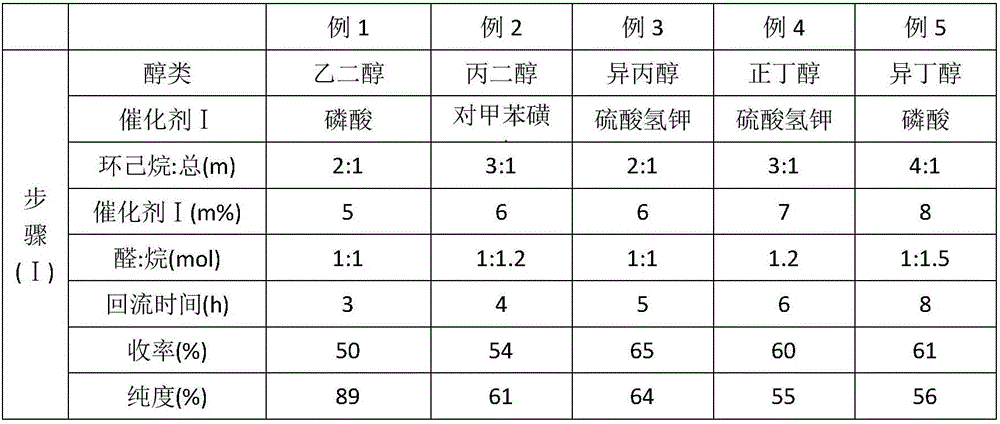
Embodiment 2~5
[0058] Embodiments 2-5 adopt the same method steps as the above-mentioned embodiment 1, and the difference is the process parameters. The reaction steps and process parameters of embodiments 2-5 are shown in Table 1. The meanings represented by the abbreviations that appear in the table are: "catalyst (m%)" refers to the mass fraction of acidic catalyst I accounting for the total reactants in step (I), and "cyclohexane: total (m)" refers to cyclohexane and total The mass ratio of reactant, " aldehyde: alkane (mol) " refers to the mol ratio of o-phthalaldehyde and hexanaphthene; " first reflux " refers to the time of tetrahydrofuran and reductant heating reflux in step (II), " secondary reflux "Refer to the time of reflux after the addition of phthalaldehyde monoacetal, "aldehyde: THF (mol)" refers to the molar ratio of phthalaldehyde monoacetal and tetrahydrofuran, "HCl:H 2 O (v) " refers to the volume ratio of hydrochloric acid solution and water " temperature A " refers to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com