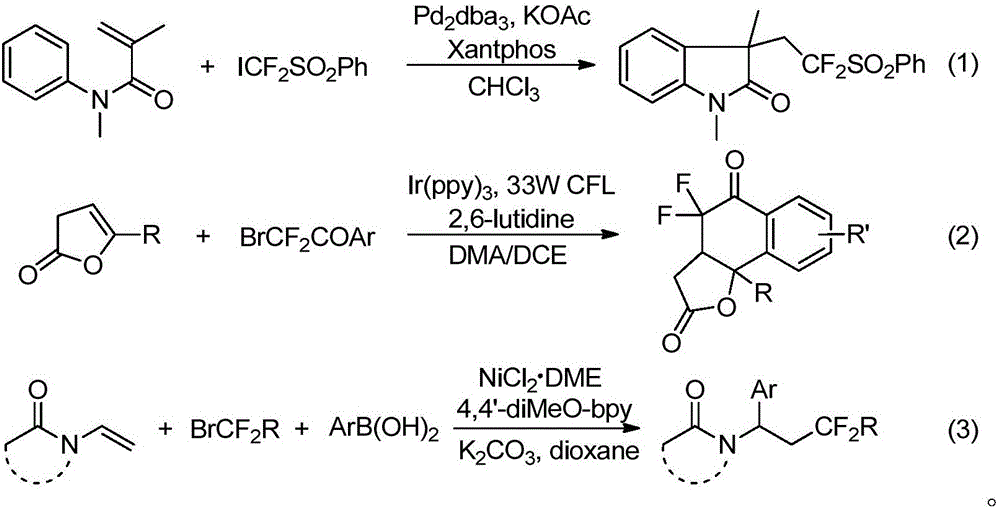
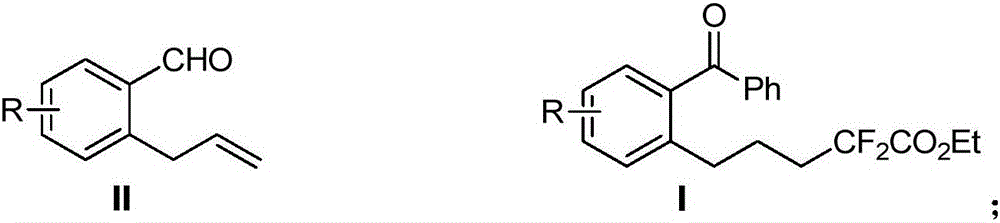
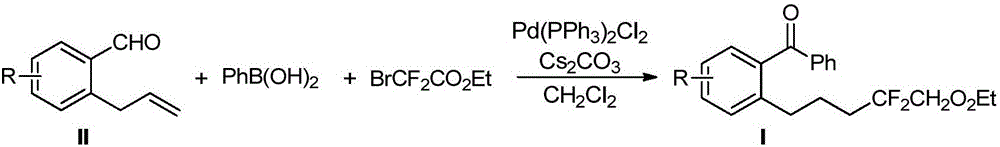Method for preparing 6-difluoro alkyl ketone
A technology of difluoroalkyl ketone and monobromodifluoroacetate, which is applied in the field of preparation of 6-difluoroalkyl ketone, can solve problems such as difficulties, and achieve improved synthesis efficiency, good reaction yield, and good application value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take a dry reaction tube, weigh allylbenzaldehyde 1a (36.5mg, 0.25mmol), phenylboronic acid 2a (37.0mg, 0.3mmol), ethyl difluorobromoacetate 3a (101mg, 0.5mmol), bis Triphenylphosphine palladium dichloride (7 mg, 0.01 mmol), cesium carbonate (122.2 mg, 0.375 mmol), and then 1 mL of dry dichloromethane were added to form a reaction system. The system was stirred at room temperature 25°C for 10 h, quenched by adding 10 mL of water, extracted three times with dichloromethane (10 mL), combined, the organic phase was washed with saturated edible water, and dried over anhydrous sodium sulfate. The organic phase was concentrated and separated by silica gel (300-400 mesh) column chromatography (eluent: petroleum ether / ethyl acetate volume ratio: 50 / 1) to obtain 70 mg of colorless liquid 4aaa, yield 81%. Product Spectrum Analysis 1 HNMR (600MHz, CDCl 3 )δ7.77–7.80(m,2H),7.55–7.62(m,1H),7.41–7.49(m,3H),7.26–7.35(m,3H),4.25(q,J=7.1Hz,2H) ,2.73(t,J=7.8Hz,2H),1.96–2.08(m,2H),1.72...
Embodiment 2
[0030] Except that the allyl benzaldehyde derivative shown in structural formula 1b is used to replace the allyl benzaldehyde shown in structural formula 1a in Example 1, all the other operating steps are the same as in Example 1, yield: 77%, colorless liquid; product Spectrum analysis 1 H NMR (600MHz, CDCl 3 )δ7.75–7.82(m,2H),7.60–7.65(m,1H),7.47–7.50(m,2H),7.38–7.44(m,1H),7.25–7.29(m,2H),4.26( q, J=7.2Hz, 2H), 2.67(t, J=7.8Hz, 2H), 1.95–2.06(m, 2H), 1.71–1.76(m, 2H), 1.30(t, J=7.1Hz, 3H ); 13 C NMR (151MHz, CDCl 3 )δ196.8,164.1(t,J=33.0Hz),140.0,138.5,137.0,133.7,131.6,131.5,130.4,130.1,128.7,128.4,116.0(t,J=250.4Hz),62.8,33.9(t,J =23.4Hz), 31.8, 23.2(t, J=4.2Hz), 13.9; 19 F NMR (565MHz, CDCl 3 )δ–105.9; HRMS (ESI) calcd for C 20 h 20 CIF 2 o 3 (M+H) + 381.1069, found 381.1081.
[0031] The reaction formula is as follows:
[0032]
Embodiment 3
[0034] Except that the allyl benzaldehyde derivative shown in structural formula 1c is used to replace the allyl benzaldehyde shown in structural formula 1a in Example 1, all the other operating steps are the same as in Example 1, yield: 81%, colorless liquid; product Spectrum analysis 1 H NMR (600MHz, CDCl 3 )δ7.74–7.79(m,2H),7.58–7.62(m,1H),7.44–7.49(m,2H),7.33(s,1H),7.26–7.26(m,2H),4.26(q, J=7.1Hz, 2H), 2.72(t, J=7.9Hz, 2H), 1.98–2.09(m, 2H), 1.72–1.81(m, 2H), 1.30(t, J=7.1Hz, 3H); 13 C NMR (151MHz, CDCl 3 )δ197.2, 164.1 (t, J = 32.9Hz), 142.6, 137.4, 136.6, 136.4, 133.5, 130.4, 130.1, 130.1, 128.5, 125.9, 116.0 (t, J = 250.3Hz), 62.8, 33.9 (t, J =23.3Hz), 32.2, 23.2(t, J=4.2Hz), 13.9; 19 F NMR (565MHz, CDCl 3 )δ–105.9; HRMS (ESI) calcd for C 20 h 20 CIF 2 o 3 (M+H) + 381.1069, found 381.1080.
[0035] The reaction formula is as follows:
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com