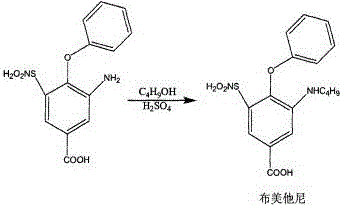
Synthetic method of bumetanide
A synthetic method and amino technology, applied in the preparation of sulfonamides, organic chemistry, etc., can solve the problems of unsuitability for industrial production, expensive deuterated reagents, complicated reaction process, etc., and achieve short reaction time, simple operation, and excellent reaction process. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The 3-amino-4-phenoxy group-5-sulfonamide benzoic acid of 0.1mol is joined in the reaction flask, then add 500ml n-butanol, stir at room temperature, then add 10mmol iron trichloride catalyst in the reaction flask, React for 6 hours. After the reaction is over, stop stirring, evaporate most of the n-butanol from the reaction solution, add 200ml of 2M sodium hydroxide solution and 100ml of ethanol, heat and reflux for 30 minutes, then add concentrated hydrochloric acid to adjust the pH to 8-8.2, and cool , stand still, suction filtration, after suction filtration, add water to the filter cake and heat to dissolve, add a small amount of activated carbon to decolorize, heat filter and cool to precipitate sodium salt, add 300ml water to heat to dissolve after suction filtration, then add concentrated hydrochloric acid to acidify to pH2- 3. Cooling, suction filtration, washing with water and drying to obtain pure bumetanide with a yield of 64.5% and a purity of 99.6%.
Embodiment 2
[0024] The 3-amino-4-phenoxy group-5-sulfonamide benzoic acid of 0.1mol is joined in the reaction flask, then add 500ml n-butanol, stir at room temperature, then add 10mmol tin tetrachloride catalyst in the reaction flask, React for 6 hours. After the reaction is over, stop stirring, evaporate most of the n-butanol from the reaction solution, add 200ml of 2M sodium hydroxide solution and 100ml of ethanol, heat and reflux for 30 minutes, then add concentrated hydrochloric acid to adjust the pH to 8-8.2, and cool , stand still, suction filtration, after suction filtration, add water to the filter cake and heat to dissolve, add a small amount of activated carbon to decolorize, heat filter and cool to precipitate sodium salt, add 300ml water to heat to dissolve after suction filtration, then add concentrated hydrochloric acid to acidify to pH2- 3. Cooling, suction filtration, washing with water and drying to obtain pure bumetanide with a yield of 86.2% and a purity of 99.4%.
Embodiment 3
[0026] Add 0.1mol of 3-amino-4-phenoxy-5-sulfonamidobenzoic acid to the reaction flask, then add 500ml of n-butanol, stir at room temperature, then add 10mmol of boron trifluoride ether catalyst to the reaction flask , reacted for 6 hours, after the reaction was finished, stop stirring, evaporate most of the n-butanol from the reaction solution, add 200ml of 2M sodium hydroxide solution and 100ml of ethanol and heat to reflux for 30 minutes, then add concentrated hydrochloric acid to adjust the pH to 8-8.2, Cool, stand still, and suction filter. After suction filtration, add water to the filter cake and heat to dissolve. After adding a small amount of activated carbon for decolorization, heat filter and cool to precipitate sodium salt. After suction filtration, add 300ml of water to heat and dissolve, then add concentrated hydrochloric acid to acidify to pH2 -3, cooling, suction filtration, washing and drying to obtain pure bumetanide with a yield of 76.8% and a purity of 99.5%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com