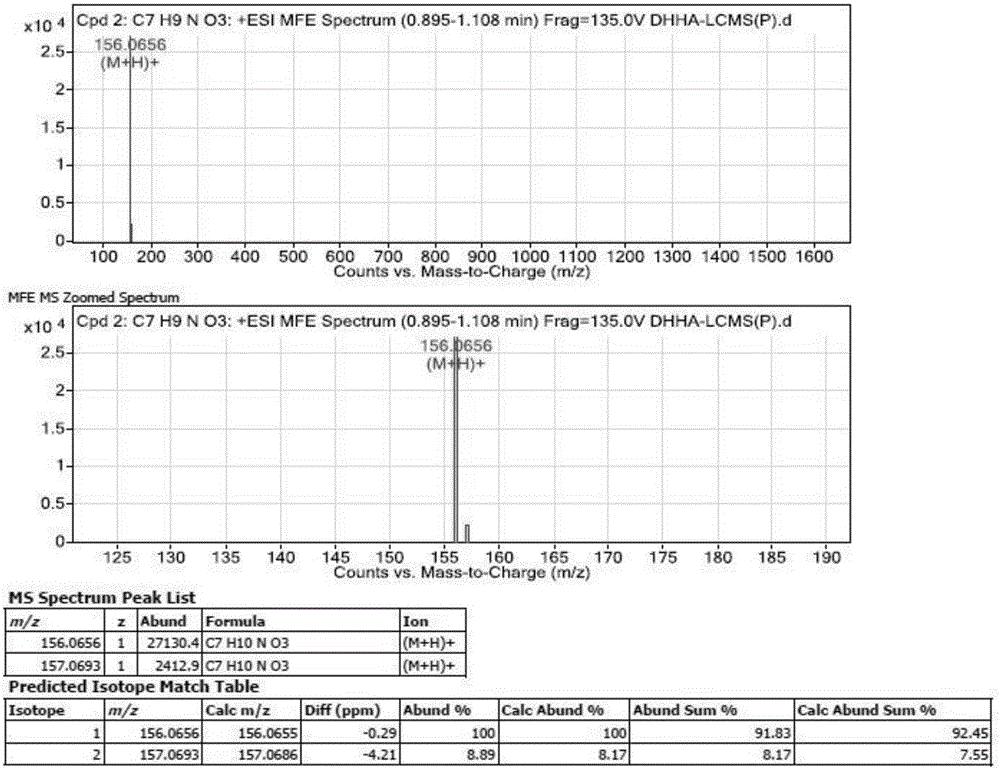
Genetic engineering strain for producing DHHA (2,3-dihydro-3-hydroxyanthranilic acid) and application thereof
A genetically engineered strain and gene technology, applied in the field of bioengineering, can solve the problems of complex metabolites and difficulties in strain transformation, and achieve the effects of simple and easy fermentation, easy separation and extraction, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
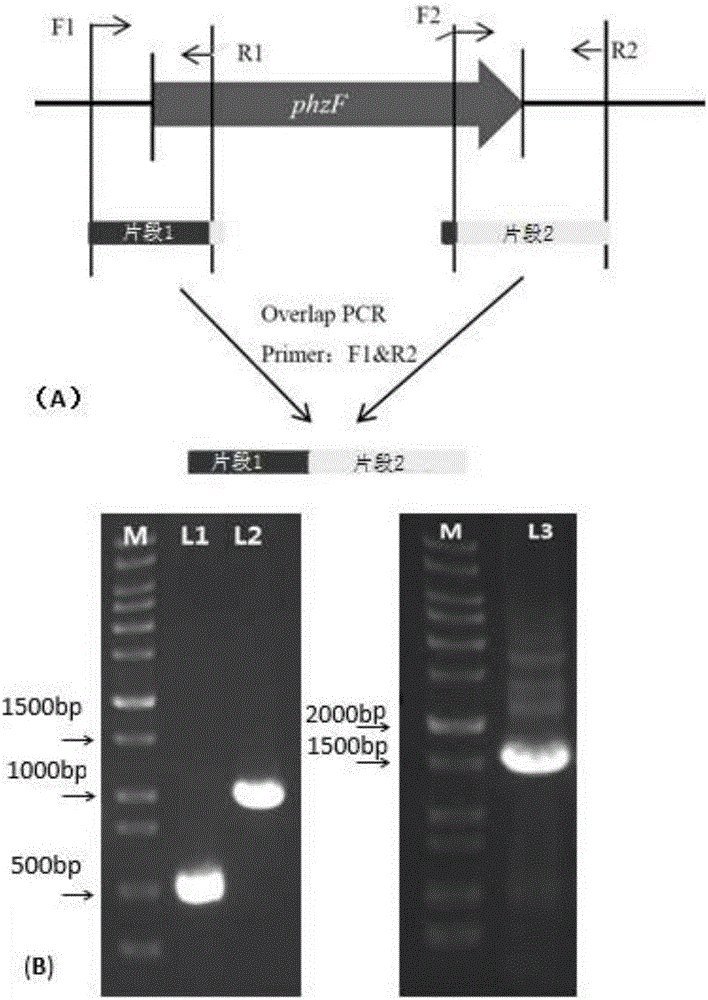
[0049] Example 1. Construction of pK18FF plasmid and construction of HT66ΔphzF mutant strain
[0050] Such as figure 1 As shown, select upstream 500bp (SEQ ID NO.3) and downstream fragments of about 1000bp (SEQ ID NO.4) on the upstream and downstream of the phzF gene, and design primers. The upstream fragment primer R1 (SEQ ID NO.5) and the downstream fragment primer F2 (SEQID NO.8) was respectively added in reverse complementarity to the opposite primer sequence, so as to facilitate the experiment of Overlap PCR. The upstream and downstream fragments were respectively amplified, and after purification and recovery, the upstream and downstream fragments were used as templates, and F1 (SEQ ID NO.7) and R2 (SEQ ID NO.6) were used as primers for Overlap PCR to obtain fusion fragments.
[0051] The successfully constructed FF fusion fragment and pk18mobsacB were subjected to double enzyme digestion, and the digested products were recovered and ligated. The ligated products were...
Embodiment 2
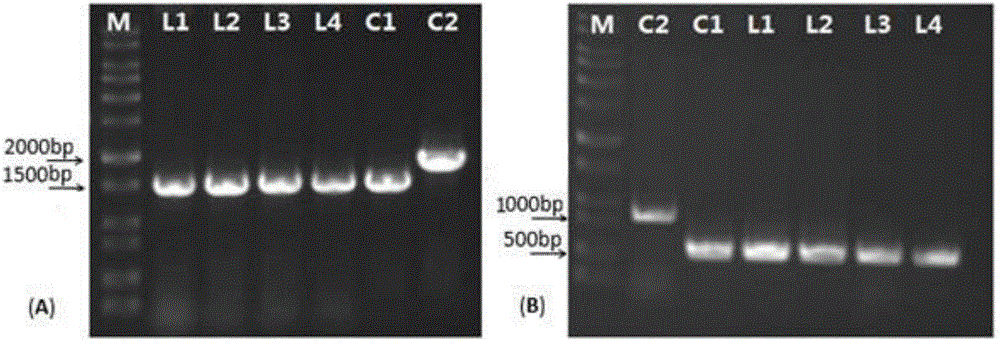
[0053] Example 2. Mutation knockout of phzF gene and construction of HT66ΔphzF mutant strain
[0054] 2.1 Construction of phzF gene in vitro mutation plasmid
[0055] Design primers phzF1 / phzF2 (SEQ ID NO.11, SEQ ID NO.12), the underlined nucleotides are restriction endonuclease Hind III and Sac I restriction sites, amplify the phzF gene fragment using the HT66 genome as a template , 1.6kb, annealing temperature 55 ℃. Using the plasmid pBS(Kan) as a template, primers pBS-Kan-R / pBS-Kan-F were designed to amplify the 1.0 kb Kanar resistance gene Kan, and the annealing temperature was 50°C.
[0056] phzF1: 5′TT AAGCTT CGGCAAGGACTACGAGGACA 3' (SEQ ID NO. 11)
[0057] phzF2: 5′AA GAGCTC CCGCCAGTACCTGAACATCAC 3' (SEQ ID NO. 12)
[0058] 2.2 Double enzyme digestion of pEX18Tc plasmid and phzF fragment
[0059] Both the double-digestion and single-digestion reactions were carried out according to the enzyme digestion system and reaction conditions provided by TAKARA.
[00...
Embodiment 3
[0113] Embodiment 3, the detection of fermentation product
[0114] Due to the high polarity of DHHA, it cannot be extracted by ethyl acetate, so the bacterial fermentation broth was filtered with 0.22 μm microporous filter paper, and then directly detected by HPLC. The HPLC instrument is Agilent 1260, phenyl column, detection wavelength 278nm, injection volume 20 μL, mobile phase 0.1% formic acid aqueous solution and methanol, gradient elution detection, the gradient is shown in Table 4 below.
[0115] Table 4
[0116] time 0.1% formic acid aqueous solution: methanol 0-6min 85∶15 7min 50∶50 17min 10∶90 18-21min 85∶15
[0117] The detection results of HT66WT and HT66ΔphzF fermentation products under different conditions are as follows: Figure 4 shown by Figure 4 It can be seen that under the detection conditions of phenazine compounds (C18 reverse column, 254nm, Figure 4 -A), phenazine-1-carboxamide was not detected in the HT66Δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com