Lytic polysaccharide monooxygenase LPMO M1 encoding gene and enzyme thereof, and preparation method and application
A technology of LPMOM1 and monooxygenase, which is applied in botany equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of low degradation efficiency in substrate crystallization area, limited efficient utilization of biomass such as cellulose, and high cost question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
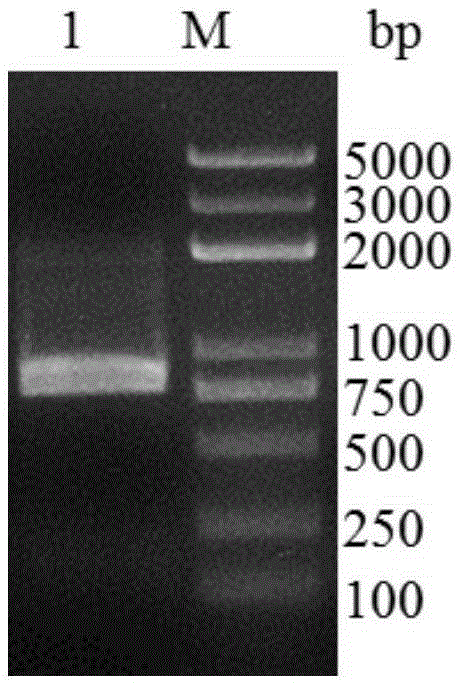
[0030] Example 1 Polysaccharide cleavage monooxygenase full-length gene cloning
[0031] Extract the RNA of Magnaporthegrisea 70-15 from the blast fungus strain Magnaporthegrisea 70-15 according to the operation steps of the fungal RNA extraction kit (Shanghai Shenggong, SK8659), and synthesize cDNA according to the operation steps of the first cDNA synthesis kit (CodeNO.: 6210A) of TaKaRa Biological Company . Primers were designed according to the nucleotide sequence of the target gene, upstream primer 5'-CTGCCCAGCCGGCGATGGCCCACTACAACTTCGAGTCCCTC-3', downstream primer: 5'-TTGTTAGCAGCCGGATCTCAGTAGTGTGCGGAGCGGCGGC-3'. PCR amplification was performed using the synthesized cDNA as a template. The PCR reaction conditions were: pre-denaturation at 98°C for 3min, denaturation at 98°C for 10s, annealing at 55°C for 5s, extension at 72°C for 2min, a total of 30 cycles, and extension at 72°C for 5min. After the PCR product was analyzed by agarose gel electrophoresis, the target fragm...
Embodiment 2
[0032] Example 2 Polysaccharide Cleavage Monooxygenase Gene Sequence Analysis
[0033]The sequencing results were analyzed using the Basic Local Alignment Search Tool (BLAST) in the GenBank database, and the Vector NTI Suite 8.0 software was used for multiple sequence alignments to analyze their homology. The domains of the sequences were analyzed using the Simple Modular Architecture Research Tool (SMART) online tool.
[0034] The coding region of the obtained polysaccharide cleavage monooxygenase gene (named lpmo M1) is 819 bp long, and its nucleotide sequence is shown in SEQ ID NO 1. Lpmo M1 encodes 271 amino acids and 1 stop codon. Its amino acid sequence is shown in SEQ ID NO 2. The theoretical molecular weight of the protein is 28.85kDa, and the predicted isoelectric point is 7.66. SMART analysis showed that the 1-21 positions in the amino acid sequence of LPMO M1 were signal peptides. The domain characteristics of LPMOM1 are more similar to those of AA9 family members...
Embodiment 3
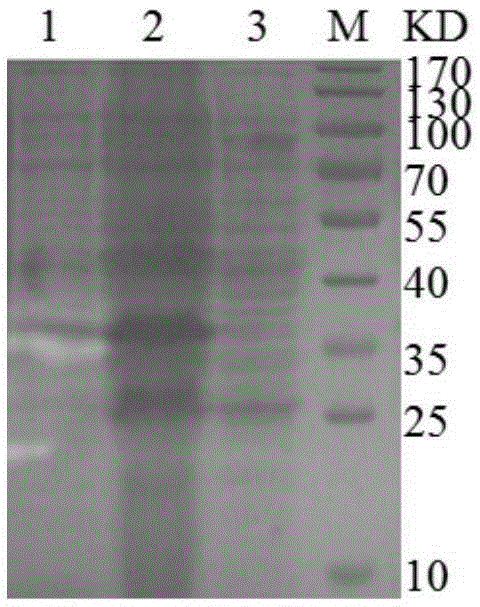
[0039] Recombinant expression of embodiment 3 lpmo M1 gene in escherichia coli
[0040] Using the genomic DNA of Magnaporthe grisea cDNA as a template, use the designed upstream primer (5'-CTGCCCAGCCGGCGATGGCCCACTACAACTTCGAGTCCCTC-3') and downstream primer (5'-TTGTTAGCAGCCGGATCTCAGTAGTGTGCGGAGCGGCGGC-3') to amplify the coding polysaccharide cleavage single plus The gene sequence of the mature protein of the oxygenase (excluding the signal peptide gene). The PCR amplification product and the expression vector pET22b (Novagen, USA) were ligated by unlimited cloning (RF cloning), and the ligated product was transformed into Escherichia coli TOP10 competent cells, and spread on Luria-Bertani medium containing 100 μg / mL ampicillin On a solid plate, culture at 37°C for 12-16 hours, pick a single clone, and use the upstream and downstream primers for colony PCR verification, and the result is an amplified product of the correct size; insert the correct single clone into a liquid cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com