Catalyst for selective oxidation of CO, and preparation method and application of catalyst
A catalyst and selective technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of narrow temperature window, difficult to meet industrial needs, etc. The steps are simple, the product purity is high, and the effect is conducive to large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Dissolve 10 mmol of calcium chloride in 100 mL of deionized water, add dropwise 5 mL of 2 mol / L aqueous sodium hydroxide solution, and stir at room temperature for 12 hours. 14 mL of 3 mg / mL H 2 PtCl 6 The aqueous solution was added to the above suspension, stirred and adsorbed for 4 hours. Add 100 mg of sodium borohydride powder, continue to stir for 12 hours, and let stand for 12 hours; wash the obtained precipitate 3 times with deionization, filter, and dry at 80°C to obtain Pt-Ca(OH) 2 The powder sample is denoted as CAT-1.
Embodiment 2
[0064] Dissolve 10 mmol magnesium chloride in 100 mL deionized water, add dropwise 10 mL aqueous sodium hydroxide solution with a concentration of 2 mol / L, and stir at room temperature for 12 hours. Add 5mL of H at a concentration of 3mg / mL dropwise 2 PtCl 6 aqueous solution and 5 mL of RuCl at a concentration of 1 mg / mL 3 The aqueous solution was added to the above suspension, stirred and adsorbed for 4 hours. Add 100 mg of sodium borohydride powder, continue to stir for 12 hours, and let stand for 12 hours; wash the obtained precipitate 3 times with deionization, filter, and dry at 80°C to obtain Pt-Ru / Mg(OH) 2 Powder samples, denoted as CAT-2.
Embodiment 3
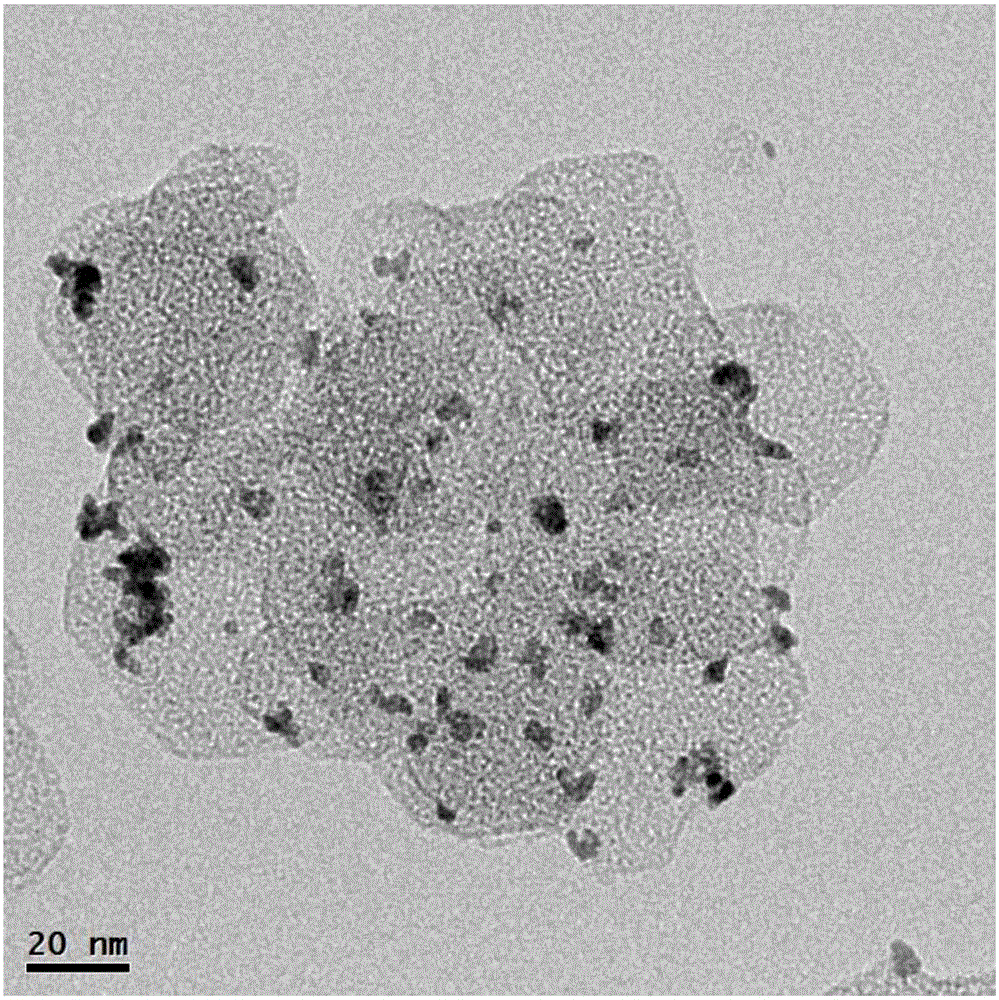
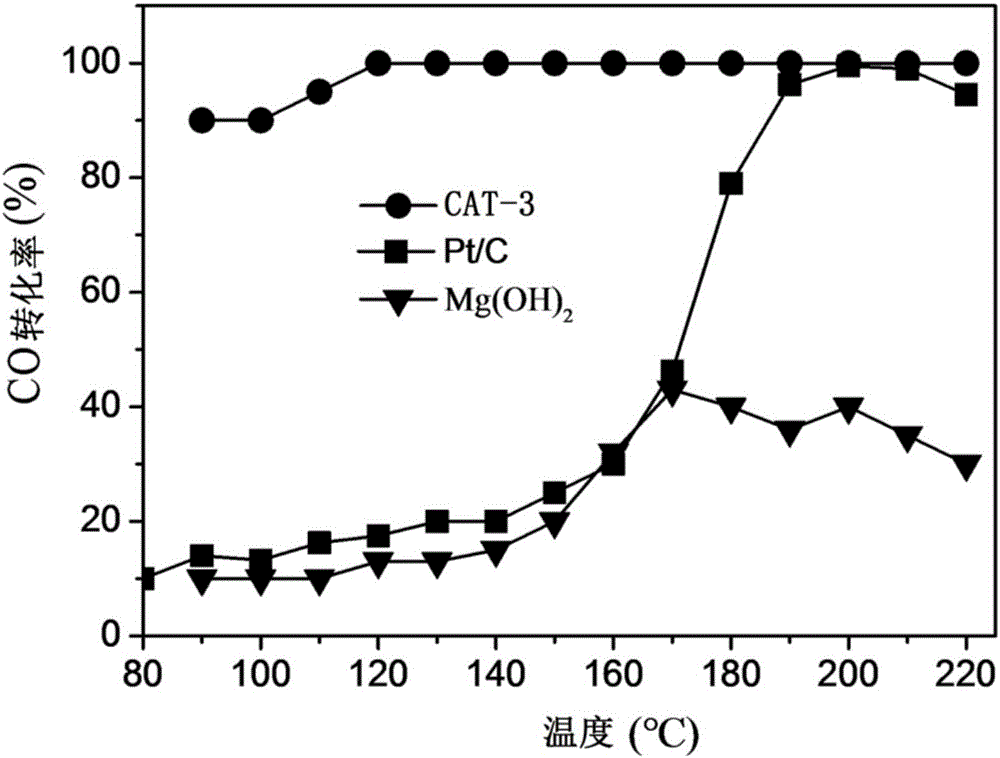
[0066] Dissolve 10 mmol of magnesium chloride in 100 mL of deionized water, add dropwise 10 mL of 2 mol / L aqueous sodium hydroxide solution, and stir at room temperature for 12 hours. Add 10mL of H at a concentration of 3mg / mL dropwise 2 PtCl 6 The aqueous solution was added to the above suspension, stirred and adsorbed for 4 hours. Add 100 mg of sodium borohydride powder, continue to stir for 12 hours, and let stand for 12 hours; wash the obtained precipitate 3 times with deionization, filter, and dry at 80°C to obtain Pt / Mg(OH) 2 Powder samples, denoted as CAT-3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com