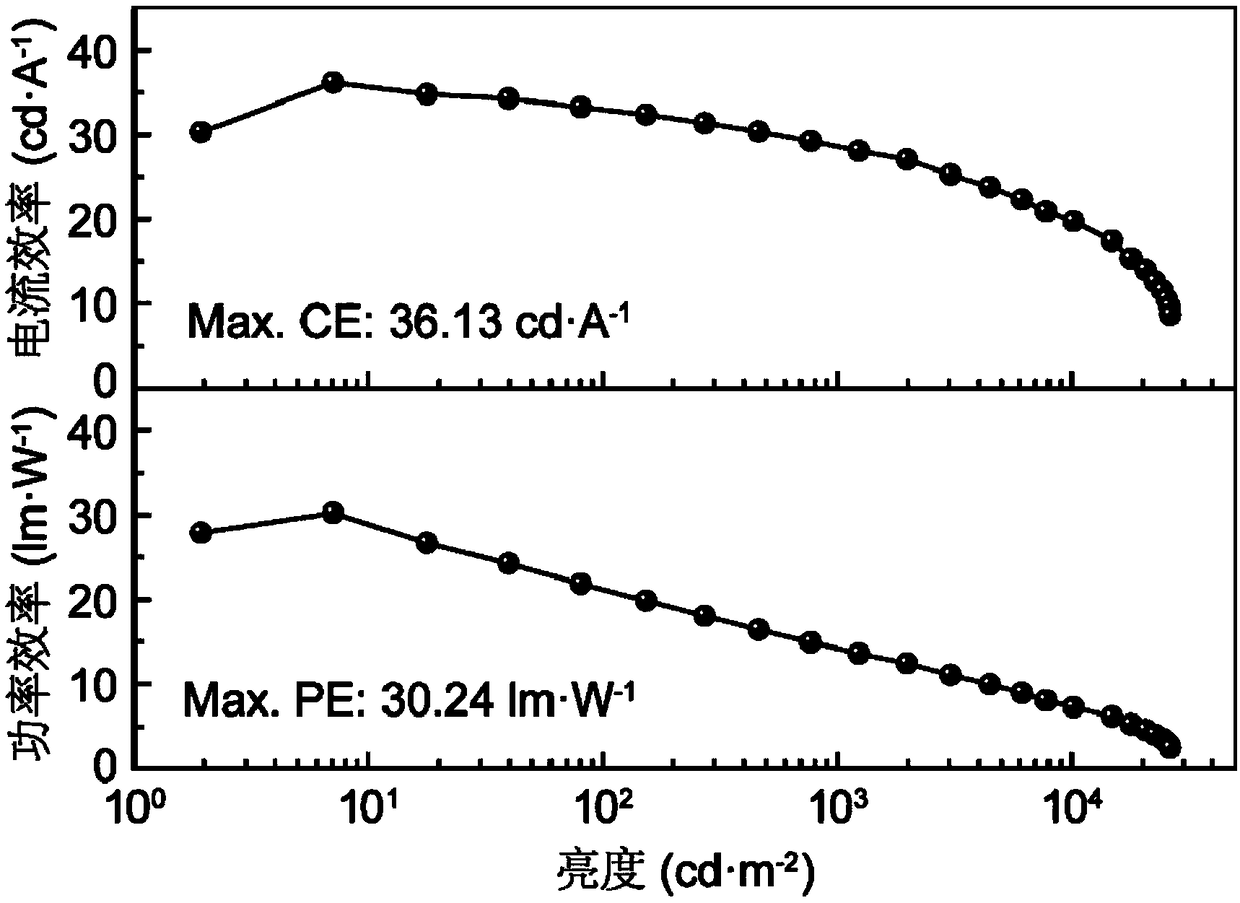
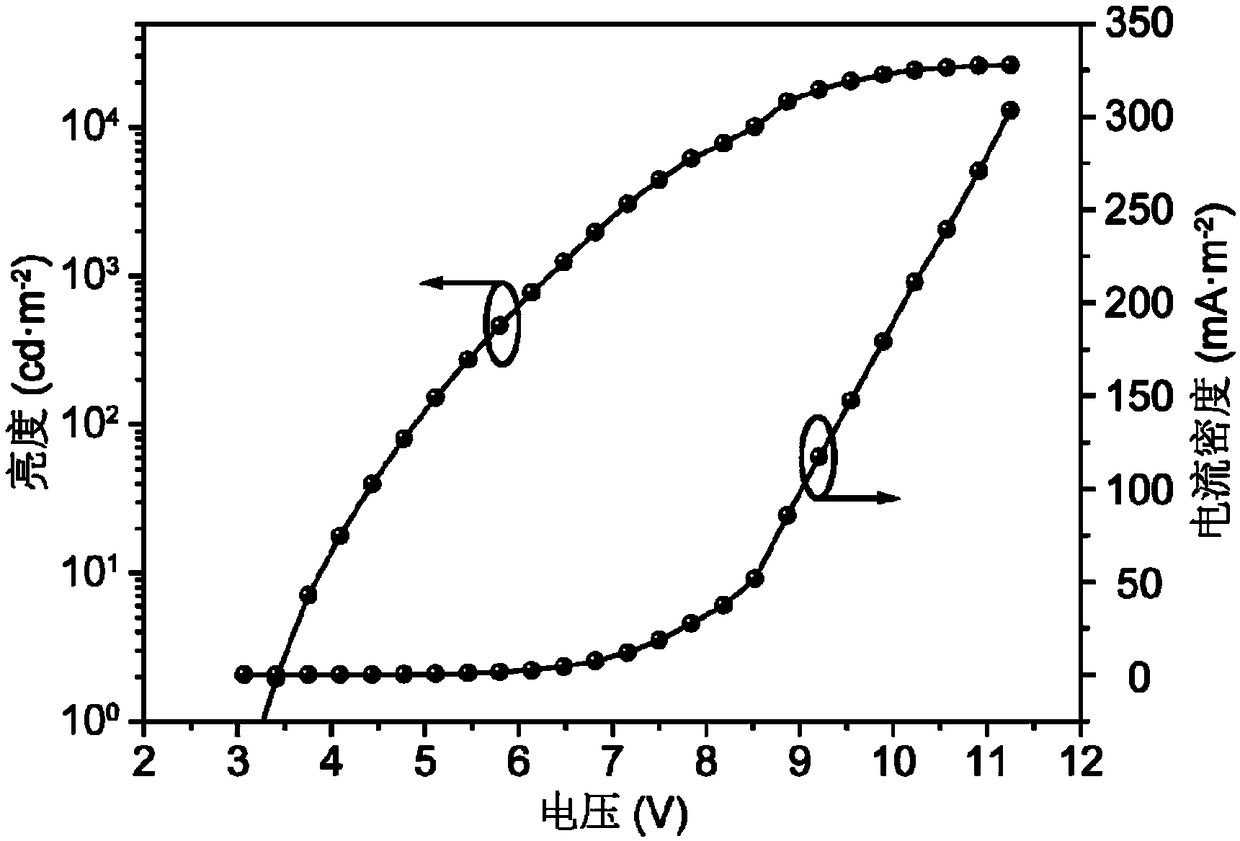
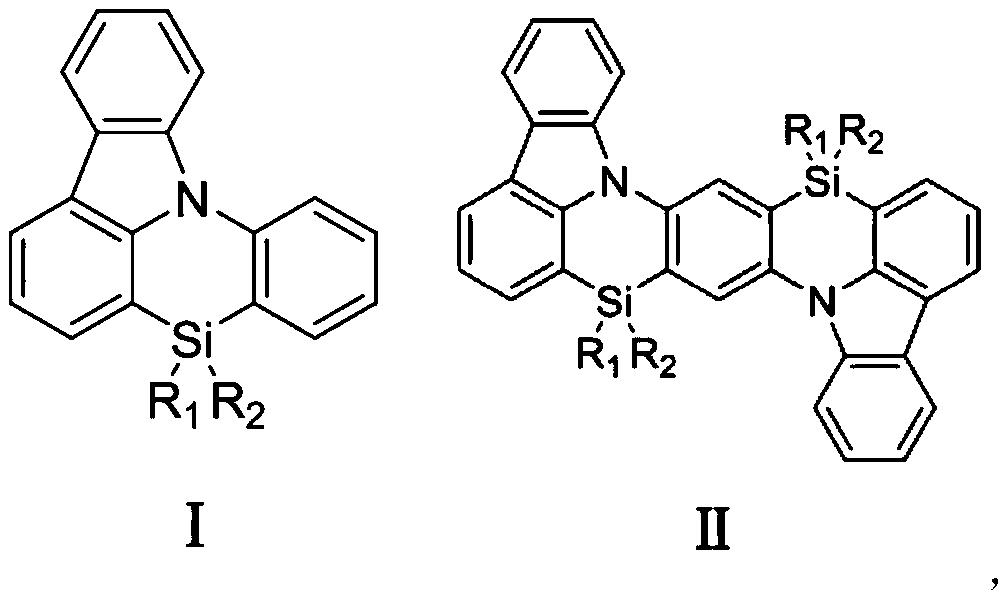
Photoelectric functional material based on silazine unit and its preparation method and application
A photoelectric functional material, silazine technology, applied in luminescent materials, chemical instruments and methods, compounds of group 4/14 elements of the periodic table, etc., can solve problems such as limiting commercial applications, and achieve improved stability and electron transport The effect of performance, low cost, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of Photoelectric Functional Material 1
[0024]
[0025] Step 1, add 10g of carbazole, 7.97g of cuprous iodide and 16.53g of potassium carbonate to the reaction bottle, vacuumize and blow nitrogen for three times, add 80mL of xylene under the protection of nitrogen, and measure 11.52mL of o-bromoiodobenzene with a syringe Add it into the reaction flask, and raise the temperature to 140° C. for 48 hours under the protection of nitrogen. The reaction solution was added with 150 mL of water, extracted with 3×150 mL of dichloromethane, and the organic phase was collected and dried over anhydrous sodium sulfate. The dichloromethane solution was spun out with a rotary evaporator. Then the crude product was dissolved in 50 mL of dichloromethane, added with silica gel powder, and the solvent was spin-dried, and purified by chromatography to obtain a bromine-containing intermediate.
[0026] Step 2, add 2g of bromine-containing intermediate to the reaction flask, ...
Embodiment 2
[0030] Synthesis of Photoelectric Functional Materials 2
[0031]
[0032] Step 1, add 10g of carbazole, 7.97g of cuprous iodide and 16.53g of potassium carbonate to the reaction bottle, vacuumize and blow nitrogen for three times, add 80mL of xylene under the protection of nitrogen, and measure 11.52mL of o-bromoiodobenzene with a syringe Add it into the reaction flask, and raise the temperature to 140° C. for 48 hours under the protection of nitrogen. The reaction solution was added with 150 mL of water, extracted with 3×150 mL of dichloromethane, and the organic phase was collected and dried over anhydrous sodium sulfate. The dichloromethane solution was spun out with a rotary evaporator. Then the crude product was dissolved in 50 mL of dichloromethane, added with silica gel powder, and the solvent was spin-dried, and purified by chromatography to obtain a bromine-containing intermediate.
[0033] Step 2, add 2g of bromine-containing intermediate to the reaction flask,...
Embodiment 3
[0037] Synthesis of Photoelectric Functional Materials 3
[0038]
[0039] Step 1, add 27.06g of carbazole, 20g of 1,4-dibromo-2,5-difluorobenzene and 61g of potassium carbonate to the reaction flask, vacuumize and blow nitrogen for three times, add 300mL of dimethyl sulfoxide under nitrogen protection, nitrogen Under protection, the temperature was raised to 150°C for 48 hours. Add 2000mL of water to the reaction solution, take the solid after suction filtration, and rinse with acetone to remove the raw material to obtain a bromine-containing intermediate.
[0040] Step 2, add 5g of bromine-containing intermediate to the reaction bottle, vacuumize, blow nitrogen repeatedly three times, add 150mL of anhydrous tetrahydrofuran (THF) under nitrogen protection, and then put the device into a dry ice / acetone bath at -78°C to cool 15min. Measure 10.6 mL of n-butyllithium n-hexane solution with a syringe, add it dropwise into the reaction flask, and react at -78°C for 1 h under ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com