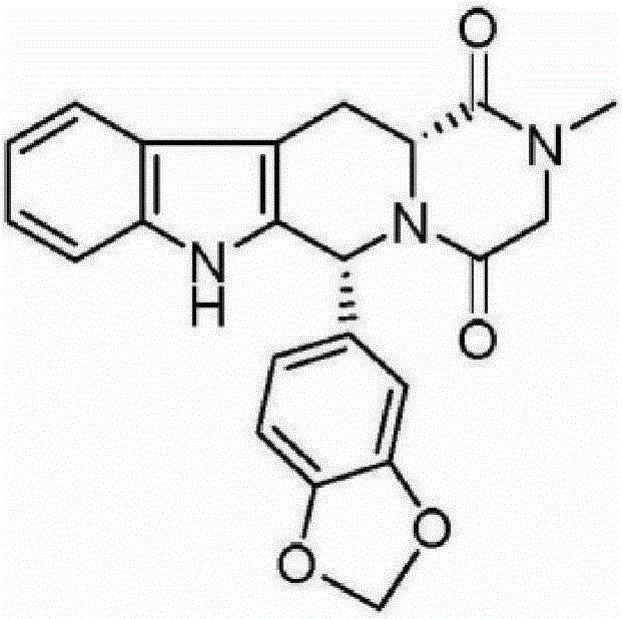
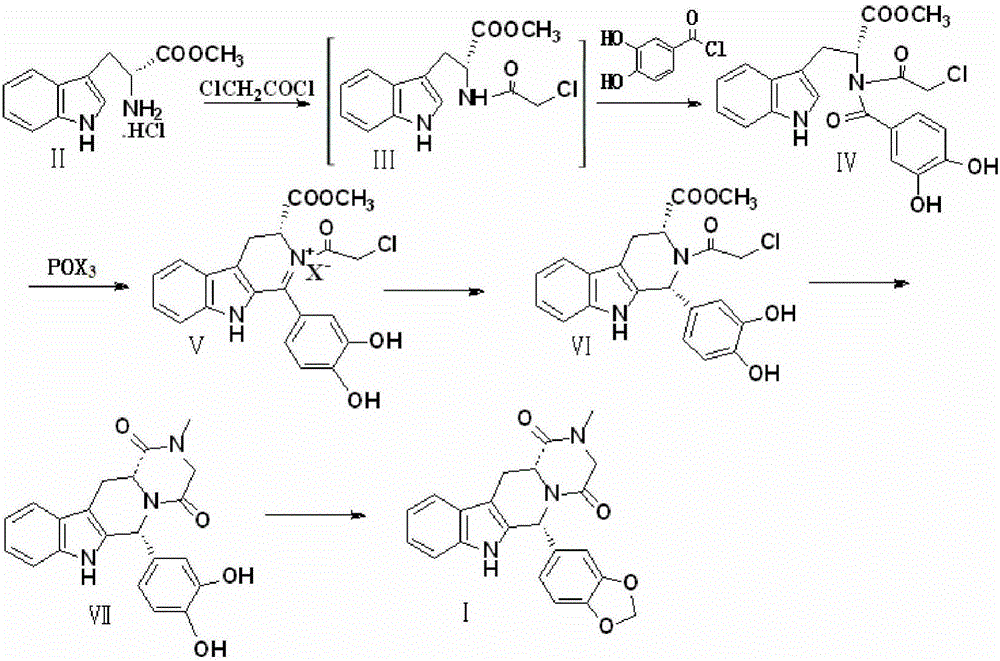
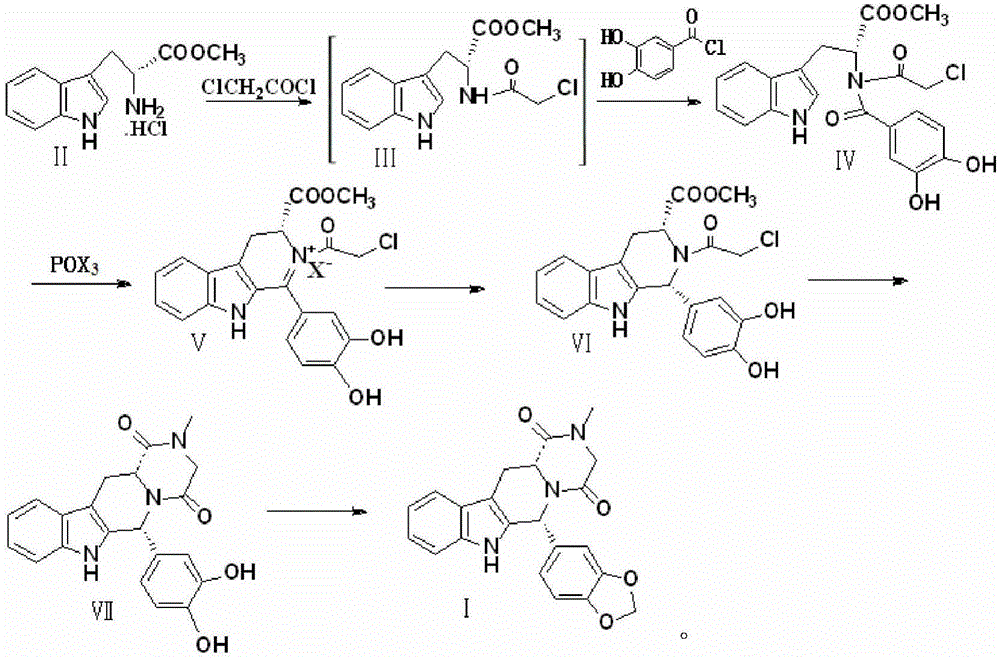
Preparation method of Tadalafil compounds
A technology of tadalafil and compounds, which is applied in the field of drug synthesis, can solve problems such as trans isomers, and achieve the effects of improving selectivity, facilitating procurement, and easy reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of compound Ⅳ
[0028] Add 25.47g of D-tryptophan methyl ester hydrochloride, 16g of NaOH, and 300ml of dichloromethane in turn into the reaction vessel, stir to dissolve, keep the temperature in the ice bath at 0-5°C, and dropwise add dichloromethane containing 11.29g of chloroacetyl chloride 20ml of methane solution was stirred for 2h to complete the reaction to obtain compound III.
[0029] Keep the temperature at 0-5°C in an ice bath, add 17.26 g of 3,4-dihydroxy-benzoyl chloride in 30 ml of dichloromethane solution to the above-prepared compound III, and stir for 2 h to complete the reaction. After the reaction, it was washed 3 times with distilled water (150ml×3), dried by adding sodium sulfate, the solvent was distilled off, and crystallized with 95% ethanol to obtain 37.92g of compound IV with a yield of 88% and a purity of 99.98%.
Embodiment 2
[0031] Preparation of compound Ⅳ
[0032] Add 25.47g of D-tryptophan methyl ester hydrochloride, 11.22g of KOH, and 300ml of dichloromethane in turn into the reaction vessel, stir to dissolve, keep the temperature in an ice bath at 0-5°C, add dropwise the solution containing 11.29g of chloroacetyl chloride 20ml of dichloromethane solution was stirred for 2h to complete the reaction to obtain compound III.
[0033] Keep the temperature at 0-5°C in an ice bath, add 17.26 g of 3,4-dihydroxy-benzoyl chloride in 30 ml of dichloromethane solution to the above-prepared compound III, and stir for 2 h to complete the reaction. After the reaction, it was washed 3 times with distilled water (150ml×3), dried by adding sodium sulfate, the solvent was distilled off, and crystallized with 95% ethanol to obtain 35.77g of compound IV with a yield of 83% and a purity of 99.96%.
Embodiment 3
[0035] Preparation of compound Ⅳ
[0036] Add 25.47g of D-tryptophan methyl ester hydrochloride, 21.2g of sodium carbonate, and 300ml of dichloromethane in turn into the reaction vessel, stir to dissolve, keep the temperature in an ice bath at 0-5°C, and add 11.29g of chloroacetyl chloride dropwise. 20ml of dichloromethane solution, stirred for 2h to complete the reaction, and compound III was obtained.
[0037] Keep the temperature at 0-5°C in an ice bath, add 17.26 g of 3,4-dihydroxy-benzoyl chloride in 30 ml of dichloromethane solution to the above-prepared compound III, and stir for 2 h to complete the reaction. After the reaction, it was washed 3 times with distilled water (150ml×3), dried by adding sodium sulfate, the solvent was distilled off, and crystallized with 95% ethanol to obtain 36.64g of compound IV with a yield of 85% and a purity of 99.96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com