Drug-loading nano fiber having sheath and containing double-core structural feature and preparation method thereof
A technology with structural characteristics and drug-loaded nanometers, which is applied in the field of materials science, can solve the problems that the slow-release effect of nanofibers cannot be effectively controlled, and achieve the effects of significant technological progress, uniform diameter distribution, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Implementation of multi-jet electrospinning process
[0026] Dissolve 8 grams of polyvinylpyrrolidone and 1 gram of ketoprofen in 100 grams of ethanol to prepare the working fluid of the sheath.
[0027] 13 grams of Eudragit E-100 and 1 gram of ketoprofen were dissolved in 100 grams of ethanol to prepare the working fluid on one side of the parallel core.
[0028] Co-dissolve 13 grams of Eudragit L-100 and 3 grams of ketoprofen in 100 grams of ethanol to prepare the working fluid on the other side of the parallel core.
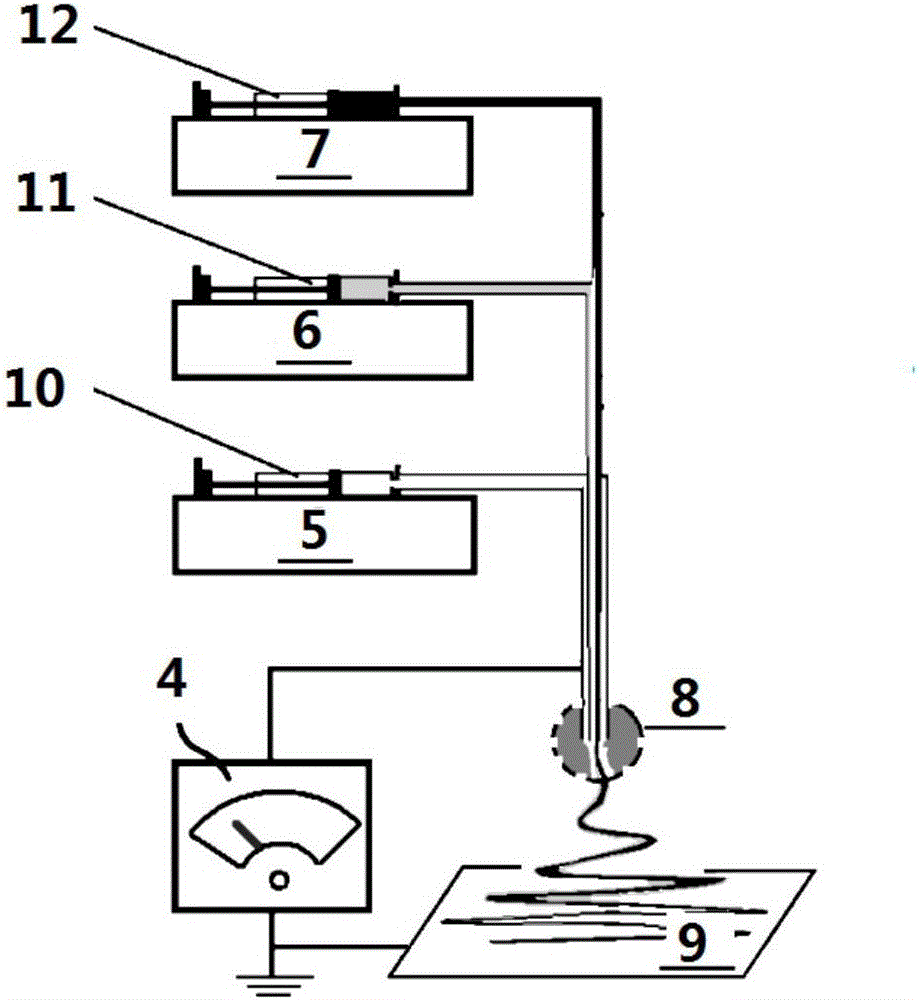
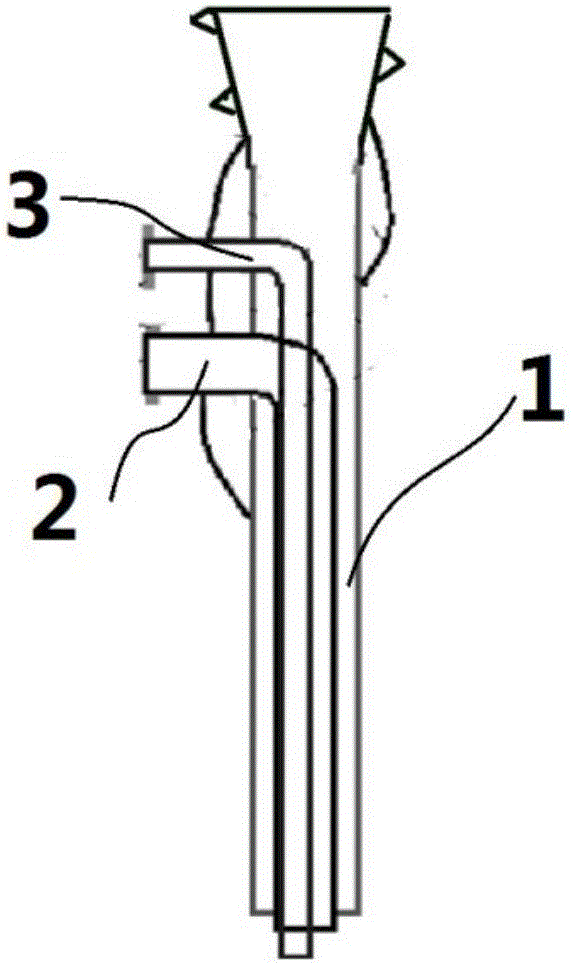
[0029] Put the above solutions into the corresponding syringes respectively, and install them on the corresponding syringe pumps, connect each working fluid to each inlet of the three-stage combined spinning head, and connect the high-voltage spinning head and the high-voltage electrostatic generator.
[0030] The high-voltage electrospinning process was implemented according to the following parameters: the flow rate of the sheath liquid...
Embodiment 2
[0033] Example 2: Characterization analysis of the morphology and structure of three-stage controlled-release electrospun drug-loaded nanofibers
[0034] Field emission scanning electron microscopy (FESEM) was used to observe the surface of the fiber prepared in Example 1 after spraying gold, and the results were as follows Figure 4 shown. The prepared fiber exhibits a good linear state, no beaded structure, smooth fiber surface and uniform fiber accumulation. The diameter is 680 ± 120 nm, the distribution is relatively uniform, and the diameter distribution is relatively concentrated.
[0035] The internal structure of the prepared fiber was observed by high-resolution transmission electron microscope (TEM), and the results were as follows: Figure 5 As shown, the sheath of the nanofiber has a clear structure of parallel double cores, including two parallel inner cores, the outer peripheries of the two inner cores are provided with an outer sheath, the outer sheath and the...
Embodiment 3
[0037] Example 3: The sustained and controlled release performance of ketoprofen provided by three-stage controlled release electrospun drug-loaded nanofibers
[0038] According to the 2015 edition of Chinese Pharmacopoeia Appendix ⅩD Release Test Method 2, the RCZ-8A intelligent dissolution tester was used to conduct the in vitro dissolution test on the drug-loaded nanofibers obtained above. The control speed is 50rpm, and the temperature is 37±0.1°C. In the first 2 hours, 900 mL of artificial gastric juice without enzymes was used as the dissolution medium, and 900 mL of artificial intestinal juice (pH6.8 phosphate buffer solution) without enzymes was used as the dissolution medium later to investigate the properties of nanofibers with drug radial isolation distribution characteristics. Controlled drug release properties in vitro. Sampling 5mL at the scheduled time, filtering through a 0.22µm microporous membrane to obtain a sample of the eluate, and immediately replenishin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com