Cobalt sulfate heptahydrate production method
A cobalt sulfate, acid-soluble technology, applied in cobalt sulfate and other directions, can solve the problems of high energy consumption, high oil content, low product pH value, etc., and achieve the effects of low energy consumption, low product oil content, and fewer processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
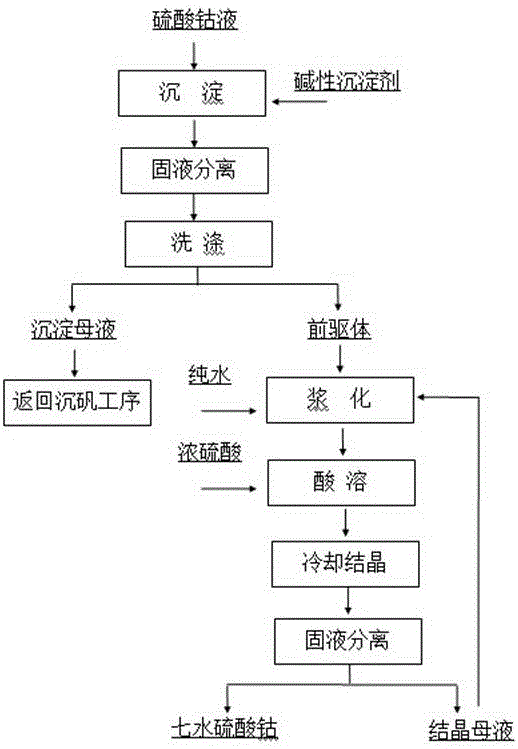
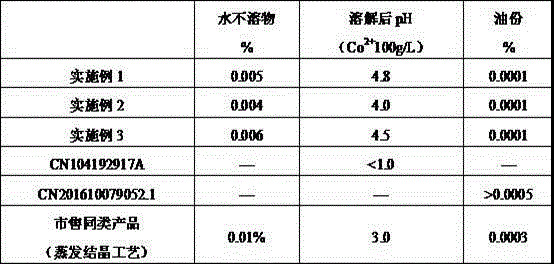
[0022] After hydrocobalt ore is leached with sulfuric acid, extracted to collect copper, precipitated alum to remove iron, P204 to remove impurities, and P507 to extract and back-extract, pure Co 2+ 120g / L cobalt sulfate solution. Add Na to pure cobalt sulfate solution 2 CO 3 For precipitation, the precipitated slurry is separated and washed by a filter press, and the precipitated mother liquor and washing water are returned to the alum sinking process of cobalt hydrometallurgy. CoCO 3 Transfer the precipitate to the dissolution tank, stir and slurry with pure water and mother liquor, and then quickly add 98% concentrated sulfuric acid for acid dissolution. The heat of reaction and dilution heat up the temperature of the acid-dissolved slurry to 106 ºC, and the pH value of the solution reaches 4.5 Stop adding concentrated sulfuric acid. Transfer the acid solution into the crystallization tank and feed circulating water to cool the crystallization, control the flow rate of ...
Embodiment 2
[0024] After the laterite nickel ore is leached with sulfuric acid, iron is removed by sinking alum, impurity is removed by P204 extraction, and pure Co is obtained by P507 extraction. 2+ 120g / L cobalt sulfate solution. NaOH is added to the pure cobalt sulfate liquid for precipitation, the precipitated slurry is separated and washed by a filter press, and the precipitated mother liquor and washing water are returned to the alum sinking process of cobalt hydrometallurgy. Co(OH) 2 Transfer the precipitate to the dissolving tank, stir and slurry with pure water and mother liquor, then quickly add 98% concentrated sulfuric acid for acid dissolution, the heat of reaction and dilution heat up the temperature of the acid-dissolved slurry to 70 ºC, and the pH value of the acid solution reaches 1.5 Stop adding concentrated sulfuric acid. Transfer the acid solution into the crystallization tank and feed circulating water to cool the crystallization, control the flow of circulating wate...
Embodiment 3
[0026] Cobalt-containing sulfide ores are subjected to oxygen pressure leaching, alum sinking to remove iron, P204 extraction to remove impurities, and P507 extraction to obtain pure Co 2+ 120g / L cobalt sulfate solution. Add NH to pure cobalt sulfate solution 4 HCO 3 For precipitation, the precipitated slurry is separated and washed by a filter press, and the precipitated mother liquor and washing water are returned to the alum sinking process of cobalt hydrometallurgy. CoCO 3 Transfer the precipitate to the dissolving tank, stir and slurry with pure water and mother liquor, then quickly add 98% concentrated sulfuric acid for acid dissolution, the heat of reaction and dilution heat up the temperature of the acid-soluble slurry to 88 ºC, and the pH value of the acid solution reaches 3.0 Stop adding concentrated sulfuric acid. Transfer the acid solution into the crystallization tank and feed circulating water to cool the crystallization. Control the circulating water flow ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com