Nickel manganese double-metal hydroxide composite material, photocatalyst and electrode material
A technology of hydroxide and composite materials, which is applied in the direction of physical/chemical process catalysts, hybrid capacitor electrodes, battery electrodes, etc., can solve the problems of capacity fading and limited development, and achieve good electrochemical cycle stability, stable structure, and electrochemical The effect of superior performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The present invention provides the preparation method of the nickel-manganese double metal hydroxide composite material described in the above technical scheme, comprising:
[0048] Dissolving bismuth oxycarbonate and alkaline substances in water to obtain a dispersion;
[0049] The dispersion liquid, the nickel salt and the manganese salt are reacted to obtain the nickel-manganese double metal hydroxide composite material.
[0050] In the present invention, preferably, bismuth oxycarbonate and a basic substance are dissolved in water and stirred to obtain a dispersion. In the present invention, the stirring is preferably magnetic stirring. In the present invention, the magnetic stirring time is preferably 8-12 minutes, more preferably 10 minutes.
[0051] In the present invention, the bismuth oxycarbonate is preferably bismuth oxycarbonate nanoflowers. The present invention has no special limitation on the source of the bismuth oxycarbonate nanoflowers, which can be p...
Embodiment 1
[0062] The preparation of embodiment 1 bismuth oxycarbonate nanoflower
[0063] Dissolve 1.66g of ammonium bismuth citrate and 0.72g of urea in 75mL of water, pour it into a 100mL high-pressure polytetrafluoroethylene reactor and stir it magnetically for 30min, react the obtained precursor at 180°C for 12h, and prepare the obtained The product is washed and dried in water and alcohol respectively to obtain the desired (BiO) 2 CO 3 nanoflowers.
[0064] The bismuth oxycarbonate nanoflowers prepared in Example 1 of the present invention are detected by SEM, and the detection results are as follows: figure 1 as shown, figure 1 For the SEM detection figure of the bismuth oxycarbonate nanoflowers prepared in Example 1 of the present invention, by figure 1 It can be seen that the bismuth oxycarbonate nanoflowers prepared in Example 1 of the present invention are regular cluster nanoflowers with stable structure and uniform size.
Embodiment 2
[0065] The preparation of embodiment 2 bismuth oxycarbonate-nickel manganese double hydroxide composite material
[0066] Disperse 50 mg of the bismuth oxycarbonate nanoflowers prepared in Example 1 in 50 mL of aqueous solution, add 300 mg of urea to adjust the alkalinity, and stir for 10 minutes with a magnetic stirrer. Then, 270 mg of nickel nitrate and 0.35 mL of manganese nitrate were added to carry out heating reaction in a water bath, the reaction temperature was 85° C., and the reaction time was 6 hours. The obtained product was washed with water and ethanol and then dried at 60° C. to obtain a bismuth oxycarbonate-nickel manganese double hydroxide composite material.
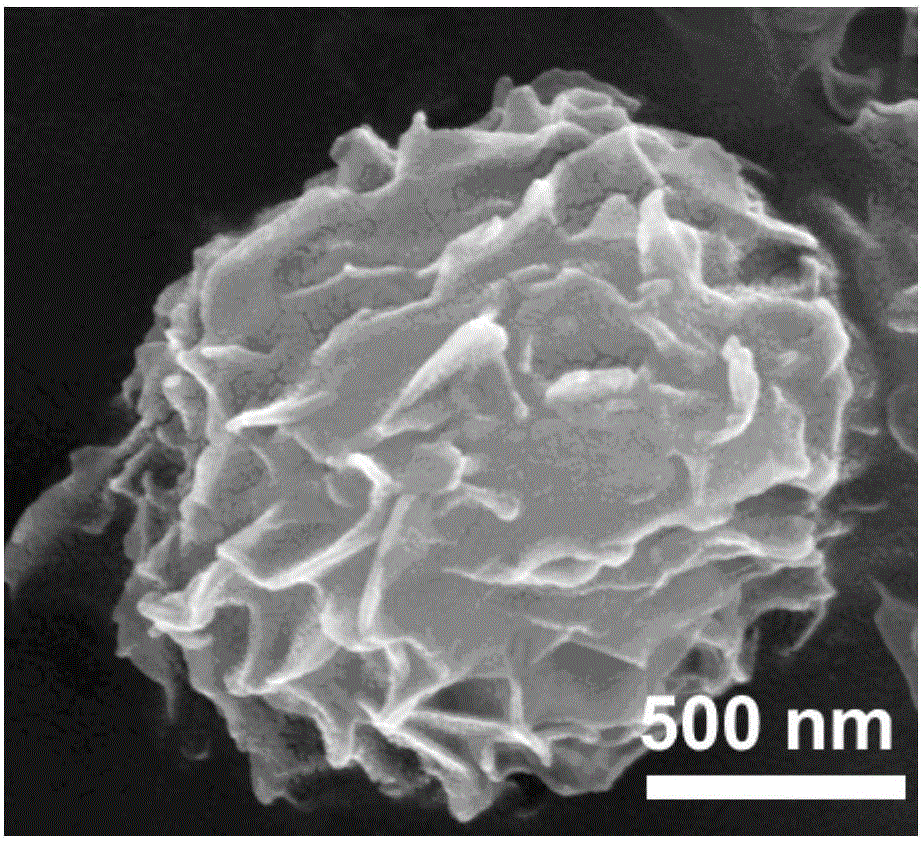
[0067] The bismuth oxycarbonate-nickel-manganese double hydroxide composite material prepared in Example 2 of the present invention is detected by SEM, and the detection results are as follows: figure 2 and image 3 as shown, figure 2 and image 3 The SEM figure of the bismuth oxycarbonate-nickel m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific capacitance | aaaaa | aaaaa |
| Specific capacitance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com