Negative electrode material for zinc-nickel battery, preparation method thereof, and battery using the same
A negative electrode material, zinc-nickel technology, which is applied in the field of zinc-nickel battery negative electrode materials and its preparation, can solve the problems of low gram capacity, poor structural stability, and reduction, and achieve the goals of reducing hydrogen evolution reaction, improving conductivity, and long cycle life Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
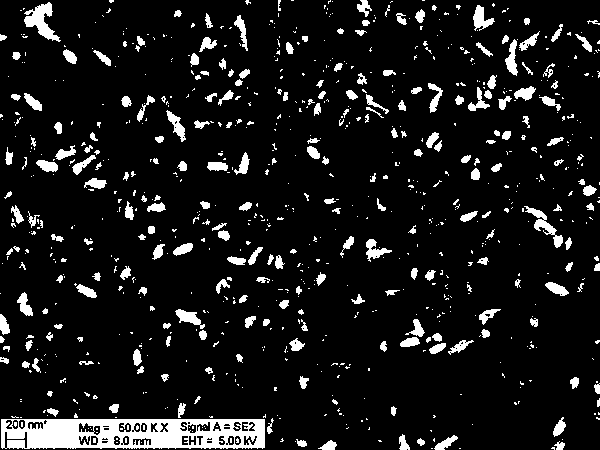
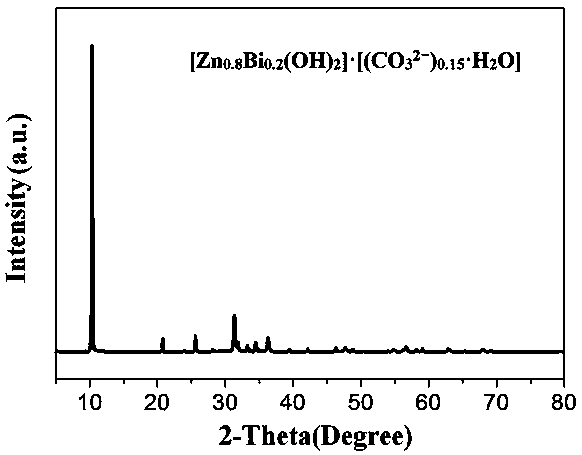
Image
Examples
Embodiment 1
[0031] [Zn 0.8 Bi 0.2 (OH) 2 ]·[(CO 3 2− ) 0.15 ·H 2 O] Preparation of anode materials
[0032] Dissolve bismuth nitrate in nitric acid solution, add zinc nitrate, in which the molar ratio of bismuth nitrate and zinc nitrate is controlled as (Zn / Bi=4), then add deionized water to prepare zinc bismuth with a molar concentration of 1.5mol / L Compound salt solution; dissolving potassium hydroxide in deionized water to prepare a potassium hydroxide solution with a molar concentration of 3mol / L; adding the potassium hydroxide solution dropwise to the zinc-bismuth compound salt solution under the reaction conditions of 25°C, continuously Stir until the pH of the mixed solution is 9 after the reaction is completed; transfer the mixed solution to a hydrothermal reaction kettle for hydrothermal treatment at 120°C for 20 hours, cool to room temperature, filter, wash, and dry to obtain a white powder; transfer the obtained white powder into the prepared sodium carbonate solution wi...
Embodiment 2
[0034] [Zn 0.6 Bi 0.4 (OH) 2 ]·[(BO 2 − ) 0.15 ·H 2 O] Preparation of anode materials
[0035] Dissolve bismuth sulfate in nitric acid solution, add zinc sulfate, wherein the molar ratio of bismuth sulfate and zinc sulfate is controlled as (Zn / Bi=3 / 2), and then add deionized water to make a molar concentration of 2.0mol / L Zinc-bismuth compound salt solution; dissolving sodium hydroxide in deionized water to prepare a sodium hydroxide solution with a molar concentration of 2mol / L; adding the sodium hydroxide solution dropwise to the zinc-bismuth compound salt solution under the reaction conditions of 30°C , stirring continuously until the pH of the mixed solution was 8 after the reaction was completed; the mixed solution was transferred to a hydrothermal reaction kettle for hydrothermal treatment at 130°C for 15 hours, cooled to room temperature, filtered, washed, and dried to obtain a white powder; the obtained white The powder was transferred to a prepared solution of ...
Embodiment 3
[0037] [Zn 0.8 Bi 0.2 (OH) 2 ]·[(BO 2 − ) 0.15 (CO 3 - ) 0.05 2H 2 O] Preparation of anode materials
[0038] Dissolve bismuth chloride in nitric acid solution, add zinc chloride, in which the molar ratio of bismuth nitrate and zinc nitrate is controlled to be (Zn / Bi=4), then add deionized water to make a molar concentration of 1.5mol / L Zinc-bismuth compound salt solution; dissolving potassium hydroxide in deionized water to prepare a potassium hydroxide solution with a molar concentration of 2 mol / L; adding the potassium hydroxide solution dropwise to the zinc-bismuth compound salt solution under the reaction conditions of 35°C , stirred continuously until the pH of the mixed solution was 10 after the reaction was completed; transferred the mixed solution to a hydrothermal reaction kettle for hydrothermal treatment at 130°C for 15 hours, cooled to room temperature, filtered, washed, and dried to obtain a white powder; the obtained white The powder was transferred to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com