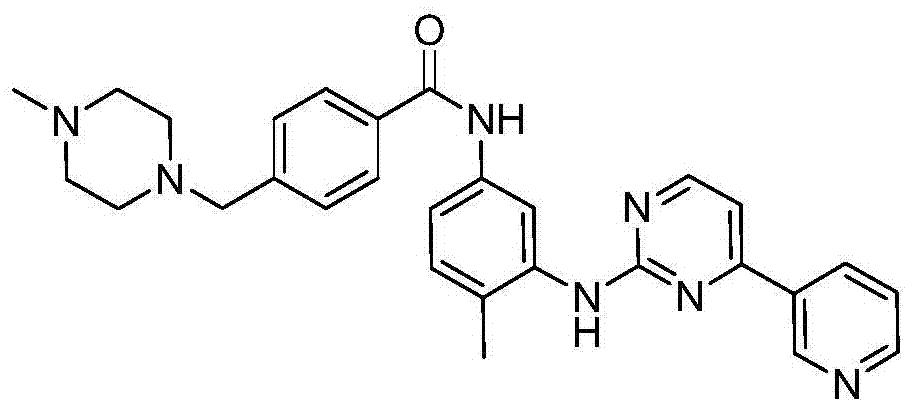
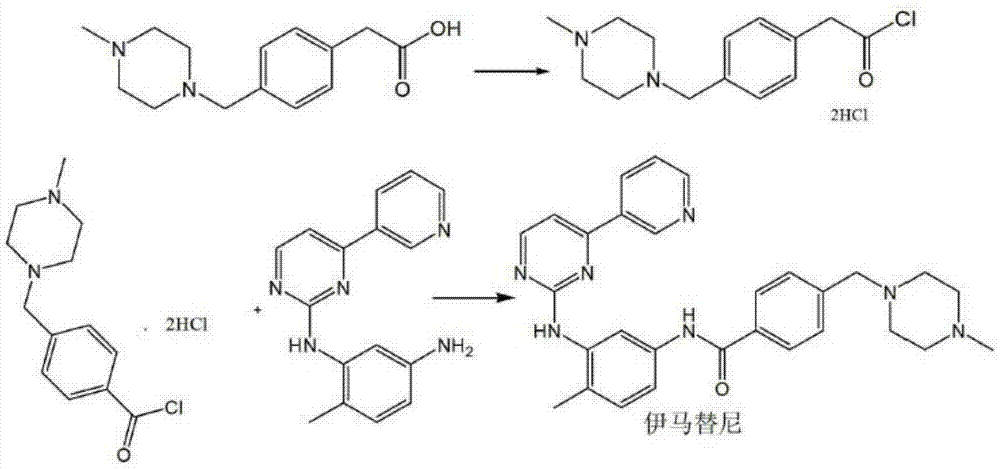
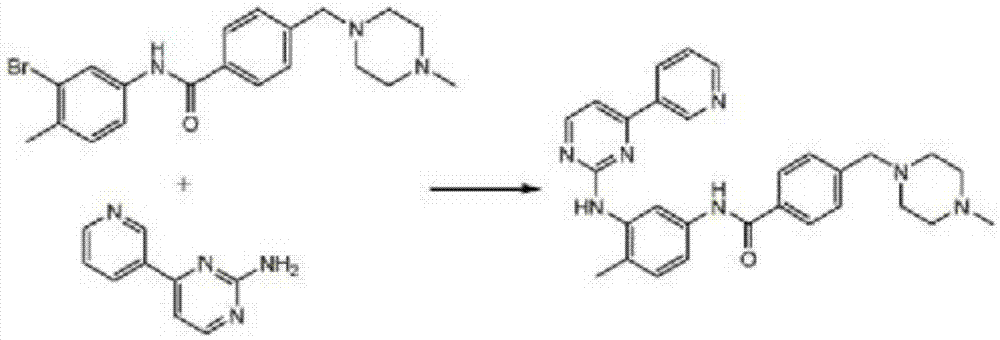
Preparation method for imatinib
A technology of methylphenyl and pyridine, which is applied in the field of imatinib preparation, can solve problems such as difficult purification, serious environmental pollution, and threats, and achieve the effects of lowering the reaction energy barrier, high product purity, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Combine 5g (12.9mmol) N-(5-iodo-2-methylphenyl)-4-(pyridin-3-yl)pyrimidin-2-amine with 3.6g (15.5mmol) 4-((4-methyl Piperazin-1-yl)methyl)benzamide was added to 90ml of dry tetrahydrofuran, and then 5.5g (25.8mmol) of potassium phosphate, 0.12g (0.65mmol) of 1,10-phenanthroline and 0.13g (0.65 mmol) cuprous iodide, stirred at room temperature in an argon atmosphere, after reacting for 4 hours, TLC point plate detection reaction was complete, filtered the reaction solution, poured the filtrate into 200ml water, precipitated solid, filtered, washed with water and acetonitrile respectively The cake was dried under reduced pressure and vacuum at 50°C to obtain 5.8 g of pale yellow-white solid imatinib with a yield of 92% and an HPLC purity of 99.4%.
Embodiment 2
[0045] Combine 25g (64.4mmol) N-(5-iodo-2-methylphenyl)-4-(pyridin-3-yl)pyrimidin-2-amine with 21.0g (90.2mmol) 4-((4-methyl Add piperazin-1-yl)methyl)benzamide to 90ml 1,4-dioxane, then add 27g (127.3mmol) potassium phosphate, 0.62g (3.2mmol) 1,10-phenanthroline and 0.13g (0.65mmol) cuprous iodide, stirred at room temperature in an argon atmosphere, after reacting for 6 hours, TLC spot plate detection reaction was complete, filtered the reaction solution, poured the filtrate into 200ml water, precipitated solid, filtered, respectively The filter cake was washed with water and acetonitrile, and vacuum-dried under reduced pressure at 50°C to obtain 26.6 g of pale yellow-white solid imatinib with a yield of 83.7% and a purity of 99.0% by HPLC.
[0046] ES-MS m / z=494[M+H + ].
[0047] 1 H NMR (DMSO-d 6 ,400MHz)δ(ppm):10.21(s,1H,NH),9.28(d,1H,4J=1.8Hz), 8.87(s,1H,NH),8.77(dd,1H,3J=4.7,4J= 1.5Hz), 8.52(d, 1H, 3J=6.9Hz), 8.47(m, 1H), 8.05(d, 1H, 4J=1.9Hz), 7.83(d, 2H, 3J=8.2Hz)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com