Preparation method of tin based catalyst, tin based catalyst and application of tin based catalyst
A catalyst, tin-based technology, applied in the field of catalysis in biomass conversion, can solve the problems of complex catalyst preparation process, difficult molecular sieve preparation, low selectivity, etc., achieve good industrial application prospects, single active site of catalyst, and space-time conversion rate high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of SBA-15: Dissolve 16g of P123 in 120g of water and 472mL of hydrochloric acid (2M) aqueous solution at 40℃. Crystallize in a hydrothermal kettle at 100°C for 24h, filter and wash with water, dry at 100°C, and roast at 550°C to obtain white powder SBA-15.
[0027] Preparation of MCM-41: Dissolve 25g of sodium silicate in a beaker with 30ml of distilled water, heat and dissolve 6.4g of cetyltrimethyl bromide surfactant (CTAB) in 20ml of distilled water, and cool to room temperature , after mixing, stir for 10 min, adjust the pH value of the mixture to 10 with sulfuric acid solution (5 mol / L), and continue stirring for 80 min, so that the solution becomes a viscous transparent gel. Then put it into a 100ml stainless steel hydrothermal kettle with a polytetrafluoroethylene liner, place it in an oven, crystallize at 130°C for 72 hours, take it out, cool it and filter it, wash until neutral, and then dry it at 90°C Overnight, the resulting semi-finished product...
Embodiment 2
[0030] Preparation of tin-based molecular sieve catalyst:
[0031] The precursor of tin: one or more of tin tetrachloride, tin dichloride, dimethyl tin, dioctyl tin, tetraphenyl tin, tributyl tin acetate, triphenyl tin, dissolved in ethanol in solution. At a certain temperature, pure silicon molecular sieves were added, stirred for 5 hours, filtered, dried and calcined at 600°C to obtain a Sn-molecular sieve catalyst.
[0032] The precursor of tin: one or more of tin tetrachloride, tin dichloride, dimethyl tin, dioctyl tin, tetraphenyl tin, tributyl tin acetate, triphenyl tin, dissolved in ethanol in solution. At a certain temperature, pure silicon molecular sieves were added, stirred for 12 hours, filtered, dried and calcined at 600° C. to obtain a Sn-molecular sieve catalyst.
[0033] The precursor of tin: one or more of tin tetrachloride, tin dichloride, dimethyl tin, dioctyl tin, tetraphenyl tin, tributyl tin acetate, triphenyl tin, dissolved in iso in propanol solutio...
Embodiment 3
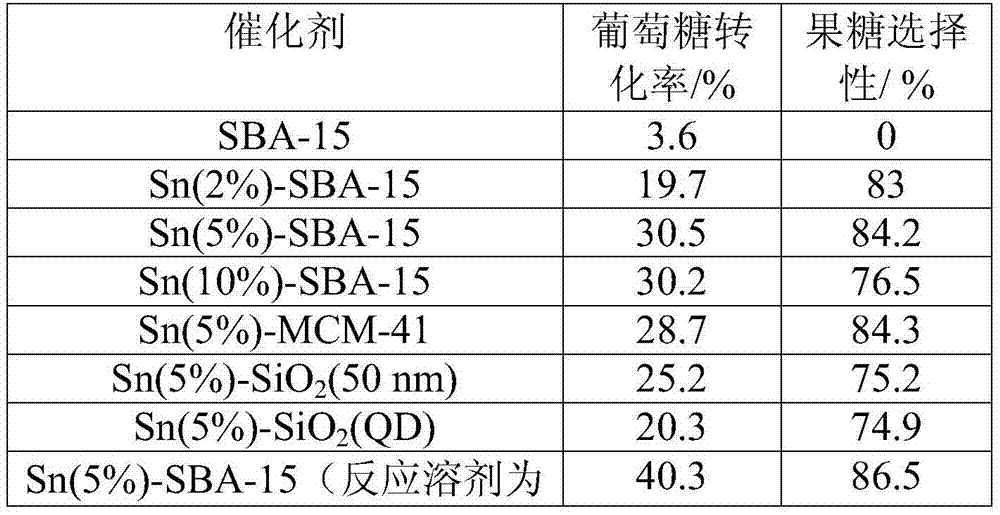
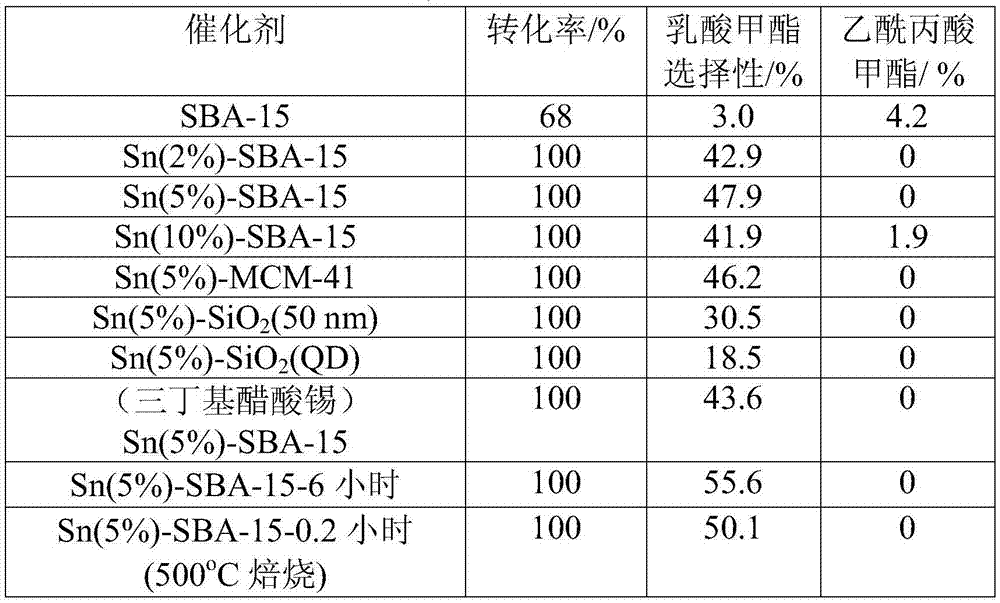
[0037] Isomerization experiment: Add 0.5g of carbohydrates, 0.15g of catalyst and 5ml of water or alcohol solution into a 20ml reaction kettle, pass through nitrogen to replace the gas three times, program the temperature to 100-120°C, and react for 5-200min. After the reaction, cool down to room temperature, take the centrifuged supernatant, separate it on a high-performance liquid chromatography calcium-type ion-exchange column and detect it with a differential refraction detector. Products such as fructose and HMF are calculated in the product yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com