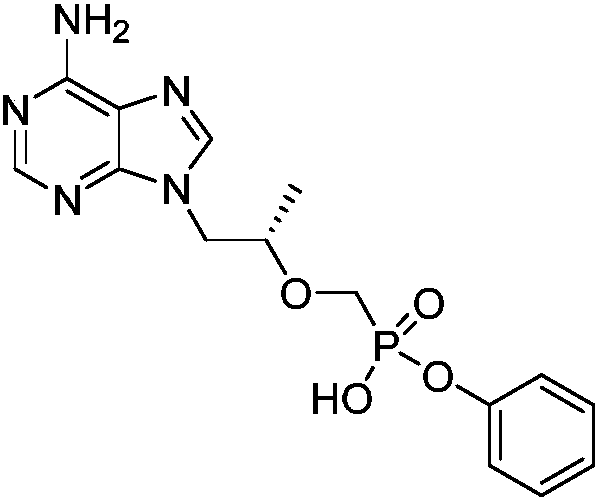
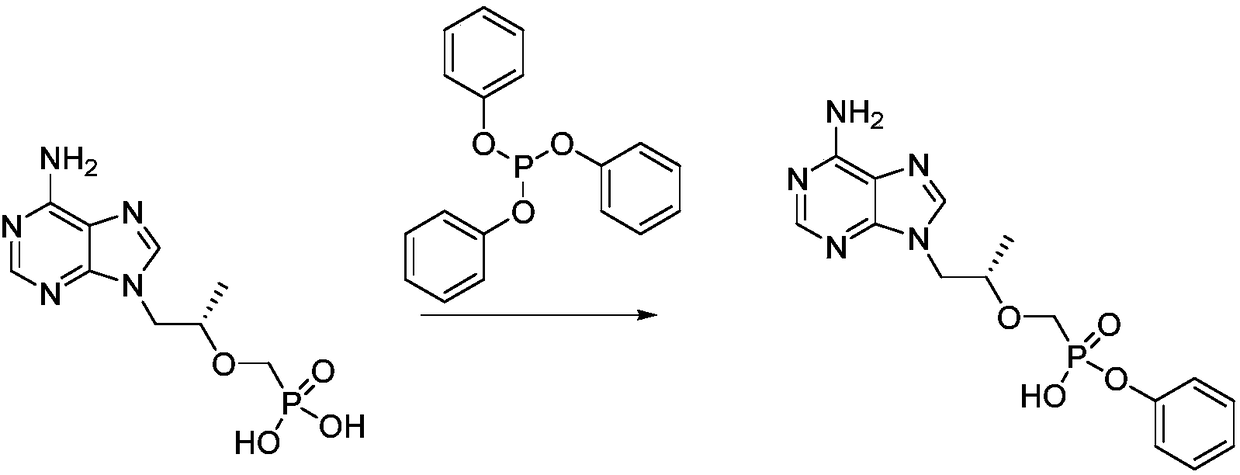
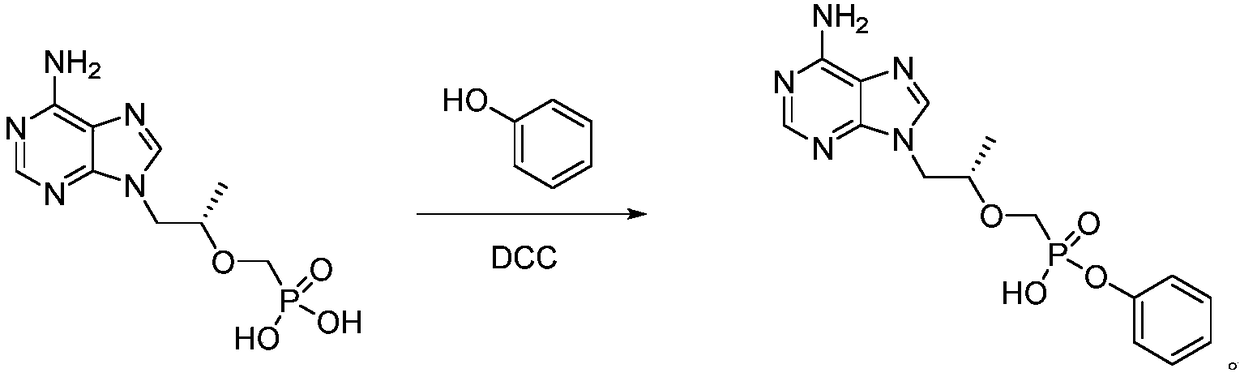
A kind of preparation method of (r)-1-(6-amino-9h-purin-9-yl) 2-phenyl ester
A purine and amino technology, applied in the field of chemical synthesis and preparation of -1-2-phenyl ester, can solve the problems of long reaction time, difficult industrialized production, low yield and the like, and achieve easily controllable conditions and easy industrialized production. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1.1 Preparation of (iodomethyl) phenyl phosphonate
[0022] Dissolve 10 g of (chloromethyl)phenyl phosphonate in 50 mL of acetonitrile, then add 9.88 g of NaI, react at 80°C for 15 hours, and the reaction is complete. The residual solid was removed by suction filtration, the solvent was evaporated to dryness under reduced pressure, 50 mL of ethyl acetate and 50 mL of water were added, and the organic layer was washed with NaS 2 o 3 The saturated solution was washed once, and the organic layer was dried and evaporated to dryness to obtain 9.76 g of the product with a yield of 73.6%.
[0023] 1 H-NMR (CDCl3, 400M) δ=3.9(d, J=3.2Hz, 2H), 7.21(m, J=8Hz, 6H), 7.36(m, J=8Hz, 4H)
[0024] MS(EI): m / e=374.02
[0025] 1.2 Preparation of phenyl (iodomethyl)phosphonate
[0026] Dissolve 10 g of phenyl (chloromethyl) phosphonate in 50 mL of DMF, then add 9.88 g of NaI, react at 80° C. for 15 hours, and the reaction is complete. The residual solid was removed by suction filtra...
Embodiment 2
[0030] 2.1 Preparation of phenyl hydrogen (iodomethyl) phosphonic acid phenyl ester
[0031] 11.7 g of phenyl (iodomethyl)phosphonate was dissolved in 60 mL of acetonitrile, and then 60 mL of 1M sodium hydroxide solution was slowly added dropwise. After the dropwise addition, the reaction system gradually became clear, and the reaction was complete after 3 hours. Evaporate to dryness under reduced pressure, add 50Ml of clear water, wash with ethyl acetate three times, adjust the pH of the ion exchange resin in the water layer to 1, evaporate the water phase, and pass through petroleum Ether: ethyl acetate = 1:1 beating and purification to obtain 7.65 g of the product with a yield of 82.08%.
[0032] 1 H-NMR (CDCl3, 400M) δ=3.4(d, J=3.2Hz,d), 7.21(m, J=8Hz, 3H), 7.4(m, J=8Hz, 2H)
[0033] MS (EI): m / e = 298.02.
[0034] 2.2 Preparation of phenyl hydrogen (iodomethyl) phosphonate
[0035] Dissolve 8.6 g of phenyl (iodomethyl)phosphonate in 60 mL of chloroform, and then slowl...
Embodiment 3
[0043] 3.1 Preparation of (R)-1-(6-amino-9H-purin-9-yl) 2-phenyl ester
[0044] Dissolve 3g of (R)-1-(6-amino-9H-purin-9-yl)propan-2-ol in 40mL of dry DMF, add 3.4g of magnesium tert-butoxide under ice-cooling, and react under nitrogen protection for 0.5 After 20 hours, 5 g of phenyl hydrogen (iodomethyl) phosphonic acid phenyl was added, and reacted under the protection of nitrogen, and the reaction was complete after 20 hours. The solvent was evaporated to dryness under reduced pressure, and water was added to adjust the pH to 2-3. After suction filtration, it was purified by beating with MeOH:H2O=1:1 to obtain 4.2 g of the product with a yield of 74.46%.
[0045] 1H-NMR (D2O, 400M) δ = 1.31 (d, J = 6.1Hz, 3H), 3.59 (dd, J = 14.0, 9.0Hz, 1H), 3.85 (dd, J = 14.0, 9.0 Hz, 1H), 4.1(m,1H),4.3(dd,J=15.0,9.0Hz,1H),4.5(dd,J=15.0,2Hz,1H),6.75(d,J=7Hz,2H),7.15(t,J =7Hz, 1H), 7.25(t, J=7Hz, 2H), 8.26(s, 1H), 8.35(s, 1H).
[0046] MS (EI): m / e = 363.31.
[0047] 3.2 Preparation of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com