A kind of conjugated polymer containing triazine group in side chain and its preparation method and application
A technology of conjugated polymers and triazine groups, applied in the field of conjugated polymers and their preparation, can solve problems such as efficiency roll-off, achieve the effects of improving performance, inhibiting concentration quenching, and simplifying the preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
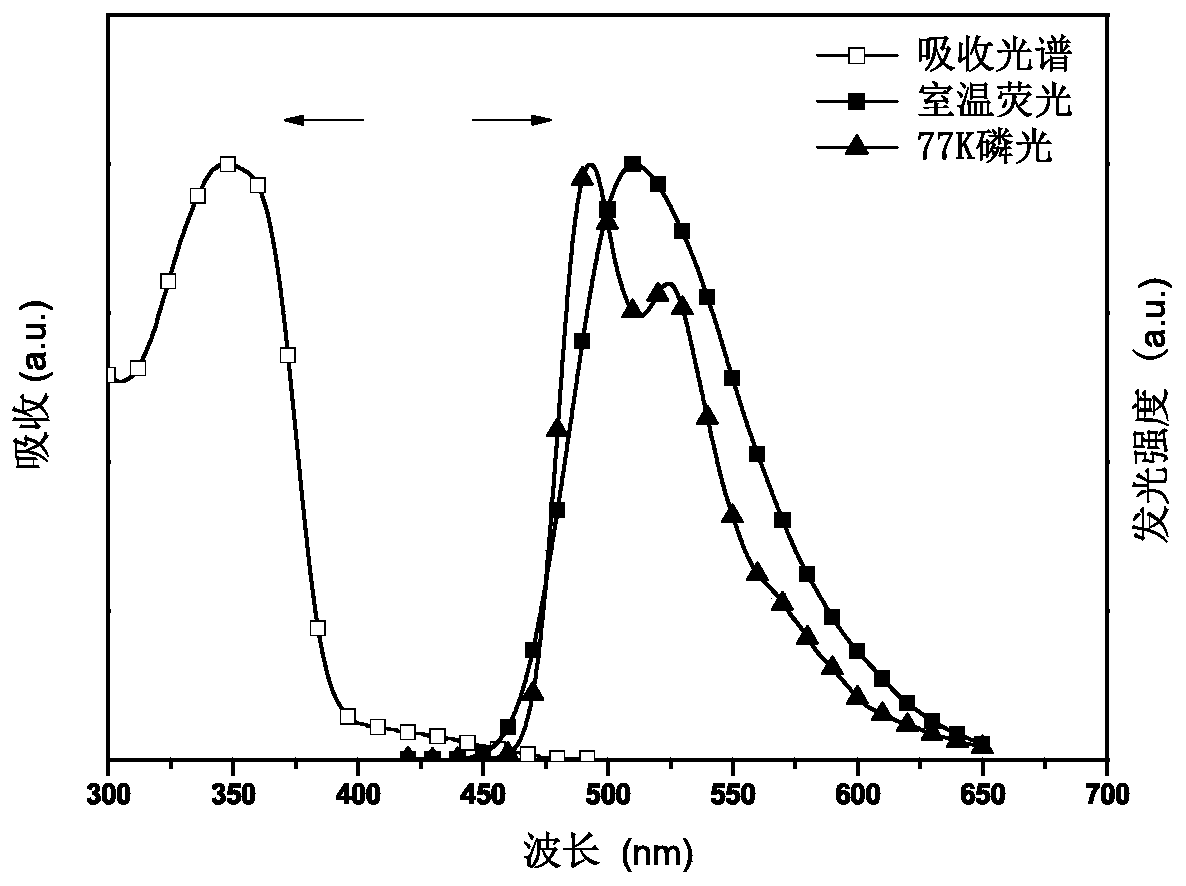
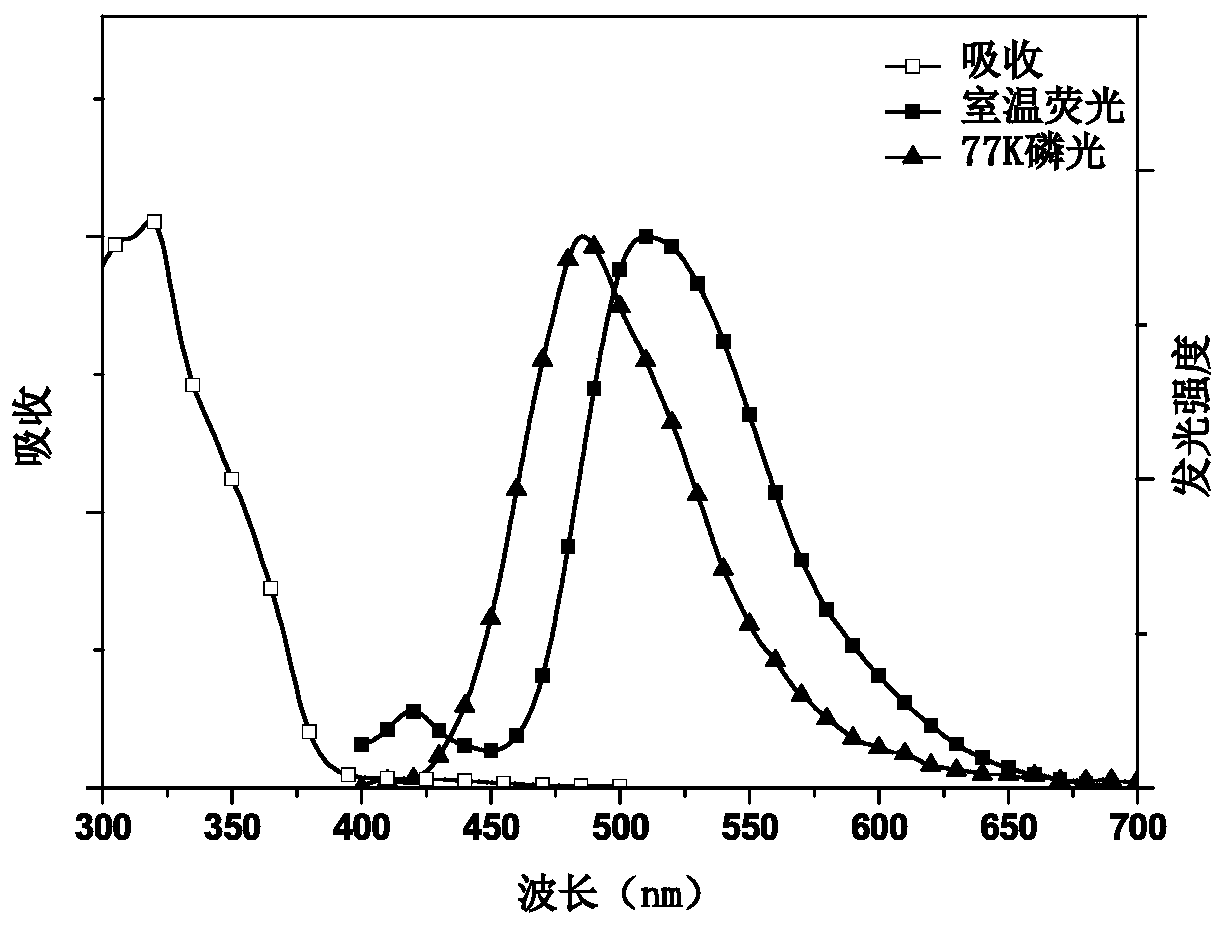
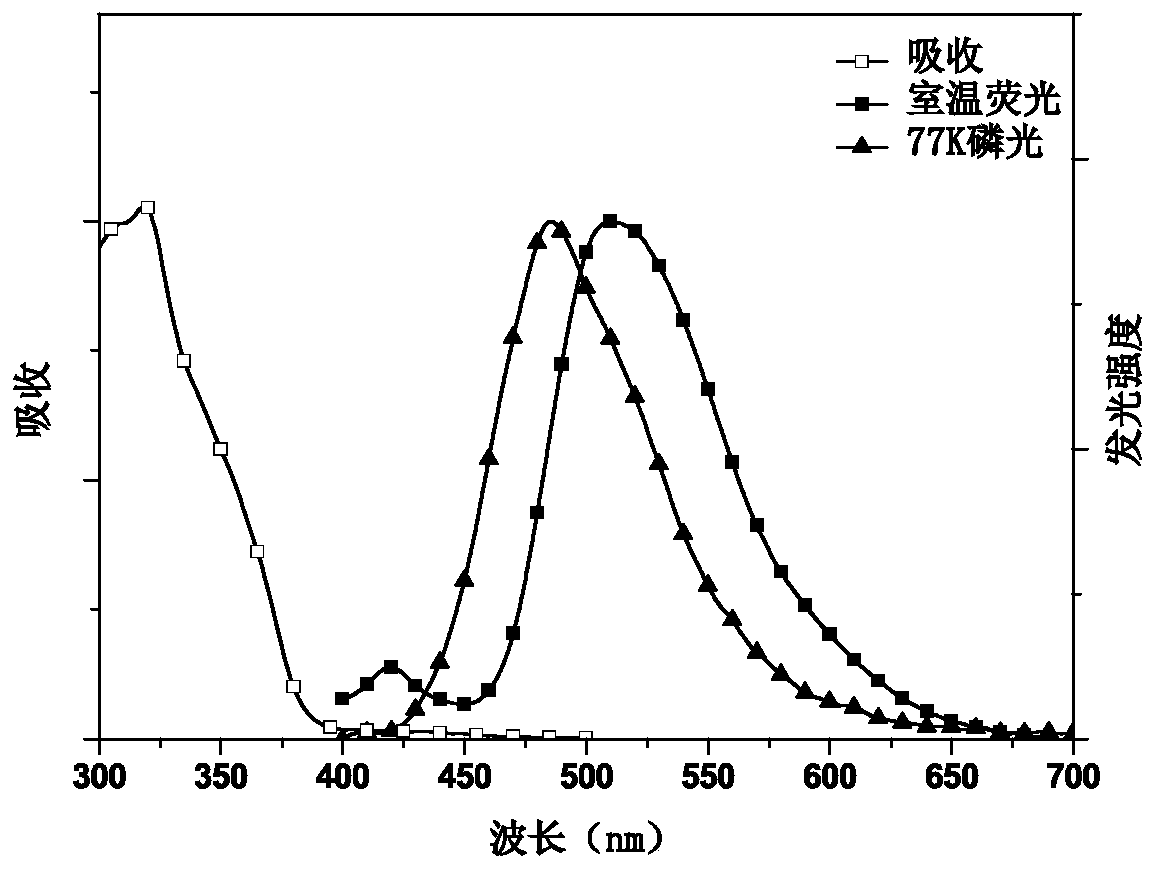
Image
Examples
preparation example Construction
[0061] The present invention also provides a method for preparing a conjugated polymer containing a triazine group in a side chain of the present invention, comprising:
[0062] Copolymerizing the compound having the structure of formula (II), the compound of formula (III) and the compound of formula (IV) to obtain the compound of the structure of formula (I);
[0063]
[0064] where R 1 , R 3 independently selected from C1-C30 alkyl, C1-C30 alkoxy, C6-C35 unsubstituted aryl or C6-C35 substituted aryl;
[0065] R 2 C1-C30 alkyl group, C1-C30 alkoxy group, C6-C35 unsubstituted aryl group, C6-C35 unsubstituted phenol group, C6-C35 substituted aryl group or C6-C35 substituted phenol group;
[0066] x is 0.001
[0067] n is 2-200.
[0068] In the present invention, the present invention copolymerizes the compound with the structure of formula (II), the compound of the structure of formula (III) and the compound of the structure of formula (IV) to obtain the compoun...
Embodiment 1
[0073] Example 1: Synthesis of polymer PCz-HAPT35%
[0074] 1) Preparation of unbrominated compound of formula (II) structure
[0075] The preparation process is as follows:
[0076]
[0077] The specific steps are:
[0078] 9,9-Dihexyl-9,10-dihydroacridine (2.0 g, 3.7 mmol), 4,6-di-tert-butyl-2-p-bromophenyl-1,3,5-triazine (1.3 g, 3.7mmol), Pd2(dba)3 (0.003g, 0.04mmol), DPPF (0.005g, 0.08mmol), sodium tert-butoxide (0.71g, 7.4mmol) were added to a 100ml three-necked flask, and 20ml of dry Toluene, ventilation, argon protection, condensation reaction at 80°C for 20h, cooled to room temperature, extracted with water and dichloromethane, the organic phase was rotary evaporated, and column separated to obtain 2.0g of product with a yield of 90%.
[0079]The obtained product was detected by nuclear magnetic resonance, and its hydrogen spectrum was: 1 H NMR (400MHz, CDCl 3 ) 68.84(d, J=8.3Hz, 2H), 7.40(d, J=8.3Hz, 2H), 7.30(m, 2H), 6.94-6.81(m, 4H), 6.19(d, J=8.8Hz, 2H), 2...
Embodiment 2
[0091] Example 2: Synthesis of polymer PCz-HAPT25%
[0092]
[0093] The specific steps are:
[0094] 2,7-Dibromo-9,9-dihexyl-10-(6-(4',6'-di-tert-butyl-1,3,5-triazine))phenyl-9,10-dihydro Acridine (0.194g, 0.25mmol), 3,6-dibromo-9-heptadecylcarbazole (0.141g, 0.25mmol), 3,6-dipinacol boronate-9-heptadecane Base carbazole (0.329g, 0.5mmol), bis(tri-o-methylphenylphosphonium) palladium dichloride (0.004g, 0.005mmol), deoxygenated potassium phosphate solution (2M, 1.6ml) were added to a 50ml single-necked flask In the middle, pump and ventilate, argon protection, add deoxygenated tetrahydrofuran (8ml), reflux at 80°C for 24h reaction; inject phenylboronic acid (0.015g, 0.1mmol) dissolved in 2ml of tetrahydrofuran into the reaction solution, react for 6h, and then add Bromobenzene (0.1ml) dissolved in 2ml of tetrahydrofuran was injected into the reaction solution and reacted for 6h; sodium diethylaminothiocarbamate (1g) dissolved in 20ml of water was added to the reaction so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com