Method for preparing poly(monothiocarbonate)
A technology of polymonothiocarbonate and carbon oxysulfide, applied in polycarbonate coatings, coatings, etc., to achieve the effect of clear structure, regular structure and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 COS / PO one-pot copolymerization synthesizes polymonothiocarbonate
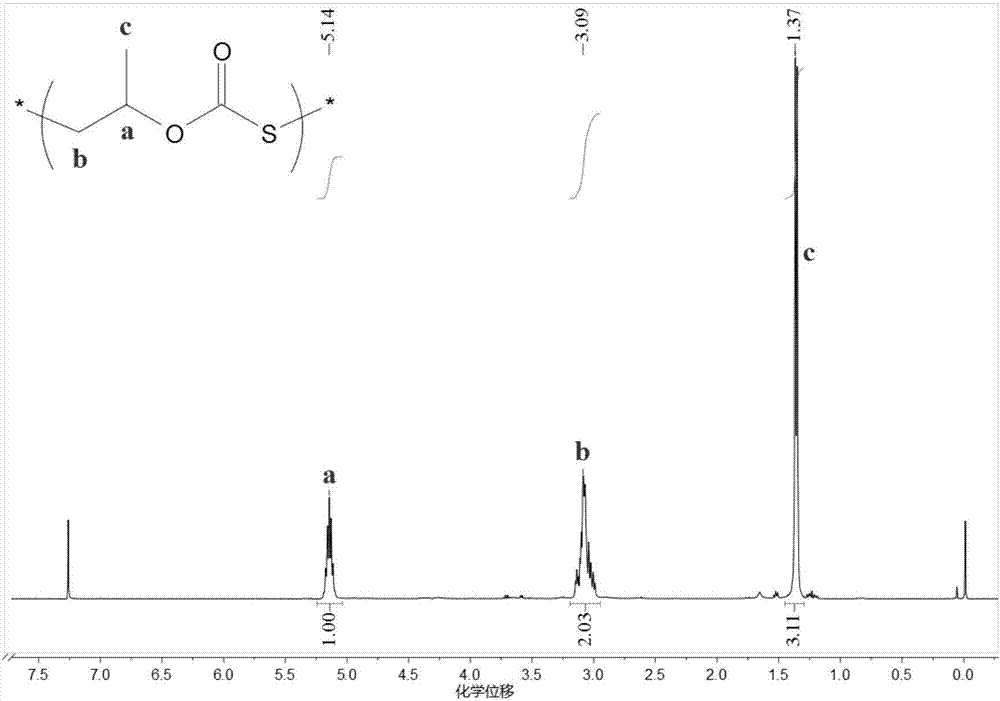
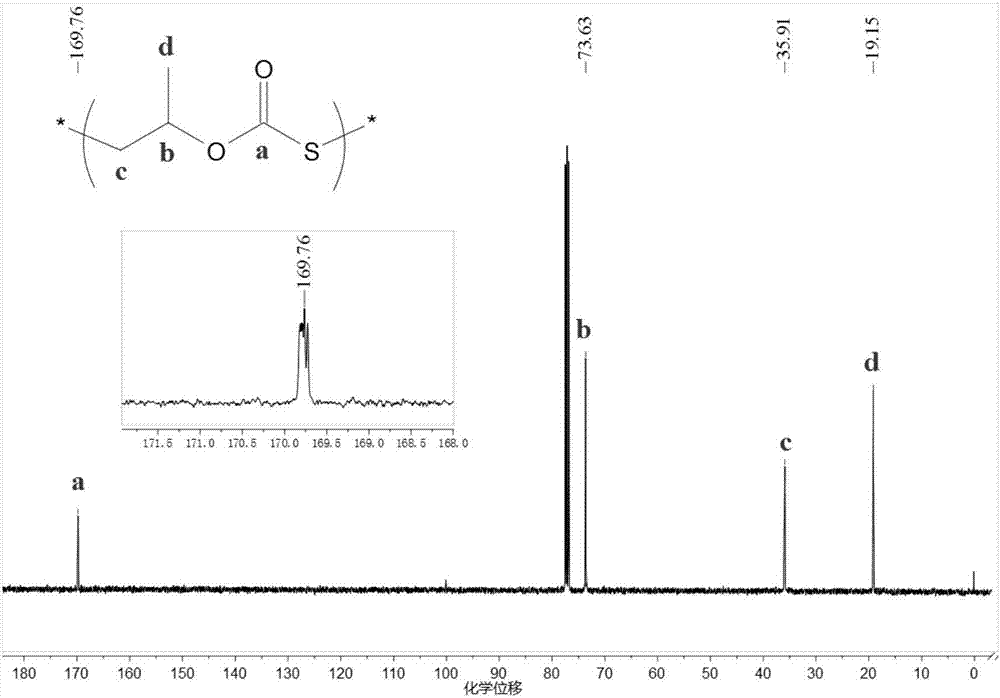
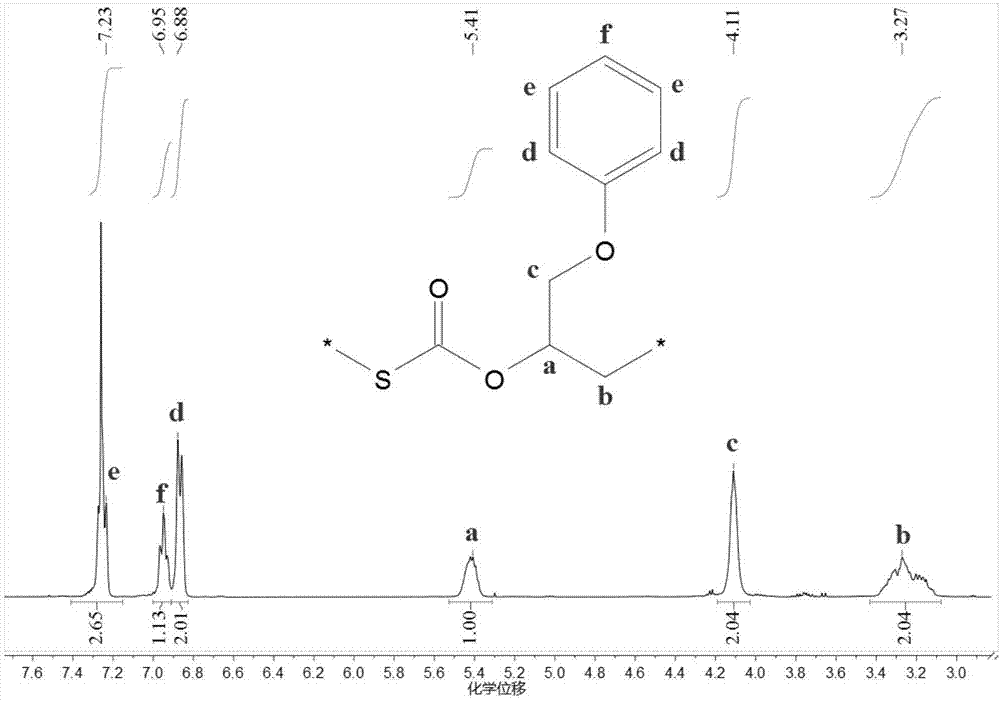
[0045] Before the polymerization reaction, remove the water in a 10mL high-pressure reactor at 110°C for about 2 hours and cool it to room temperature in a desiccator; add several quantities of initiator 1 bis(triphenylphosphoryl) chloride to the reactor in sequence Ammonium chloride ([PPN]Cl) and triethylboron (TEB), the molar ratio of the two is 1:2; the molar ratio of initiator 1 and propylene oxide (PO) is 1 / 1000; add COS, PO ( COS to PO molar ratio 2:1) and 3 mL tetrahydrofuran (THF). Then the autoclave was closed and placed in an oil bath at 25°C for 8 hours under autogenous pressure. Cool to room temperature after the reaction, first dissolve the crude product with tetrahydrofuran, then precipitate the polymer in a mixture of ethanol / deionized water / hydrochloric acid, repeat washing three times, and vacuum-dry to constant weight. The content of each chain segment in the polymer was...
Embodiment 2
[0047] Example 2 One-pot copolymerization of COS / PO to synthesize polymonothiocarbonate
[0048] Before the polymerization reaction, the 10mL autoclave was dehydrated at 110°C for about 2 hours and cooled to room temperature in a desiccator; several quantities of initiator 2 dodecyltrimethylammonium bromide were added to the autoclave in sequence (DTMeAB) and triethylboron (TEB), the molar ratio of the two is 1:1; the molar ratio of initiator 2 and propylene oxide (PO) is 1 / 500; add COS, PO (COS and PO molar ratio 2:1) and 3 mL tetrahydrofuran (THF). Then the autoclave was closed and placed in an oil bath at 60°C for 6 hours under autogenous pressure. Cool to room temperature after the reaction, first dissolve the crude product with tetrahydrofuran, then precipitate the polymer in a mixture of ethanol / deionized water / hydrochloric acid, repeat washing three times, and vacuum-dry to constant weight. The content of each chain segment in the polymer was calculated by proton nucl...
Embodiment 3
[0049] Example 3 One-pot copolymerization of COS / PO into polymonothiocarbonate
[0050] Before the polymerization reaction, the 10mL autoclave was dehydrated at 110°C for about 2 hours and cooled to room temperature in a desiccator; several quantities of initiator 3 tetrabutylammonium chloride [N(iBu ) 4 Cl] and triethylboron (TEB), both molar ratio is 1:0.1; The molar ratio of initiator 3 and propylene oxide (PO) is 1 / 500; Add COS, PO again (COS and PO molar ratio 2 : 1) and 3 mL tetrahydrofuran (THF). Then the autoclave was closed and placed in an oil bath at 120° C. for 4 h under autogenous pressure. Cool to room temperature after the reaction, first dissolve the crude product with tetrahydrofuran, then precipitate the polymer in a mixture of ethanol / deionized water / hydrochloric acid, repeat washing three times, and vacuum-dry to constant weight. The content of each chain segment in the polymer was calculated by proton nuclear magnetic spectroscopy, and the molecular wei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com