Synthesis method of N-type conjugated polyelectrolyte based on electron deficiency heterocyclic ring main chain
A technology of conjugated polyelectrolyte and synthesis method, which is applied in the field of compound synthesis and can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
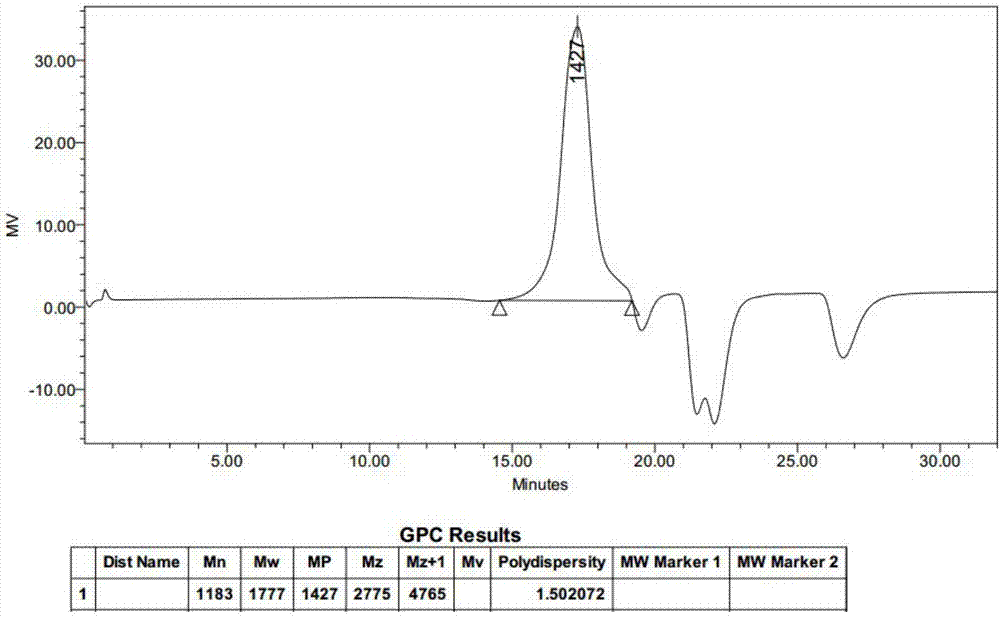
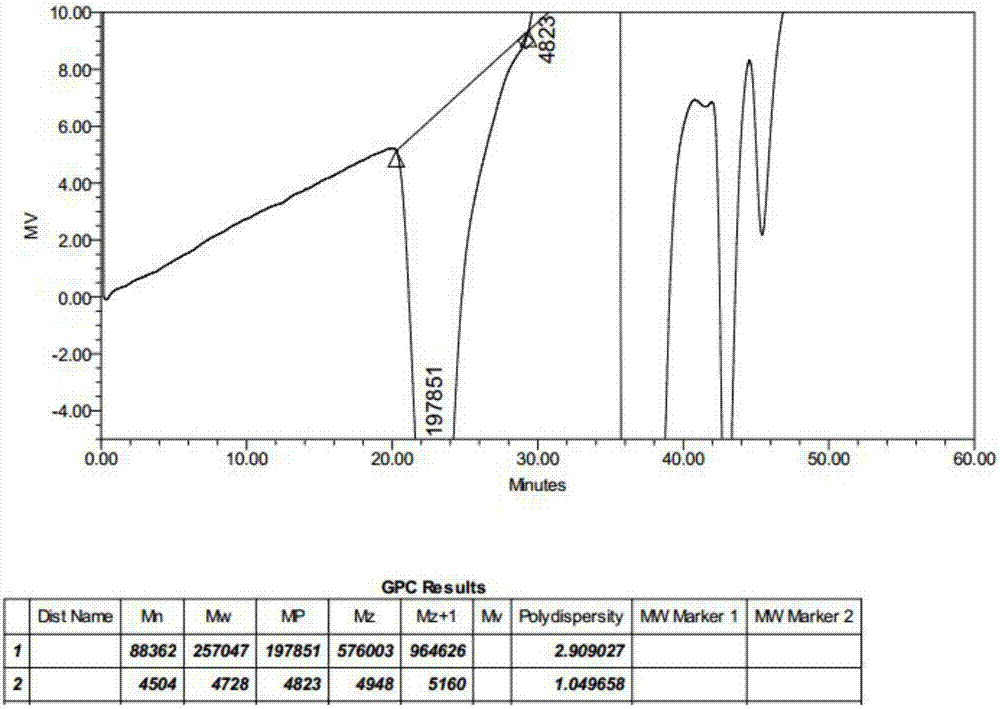
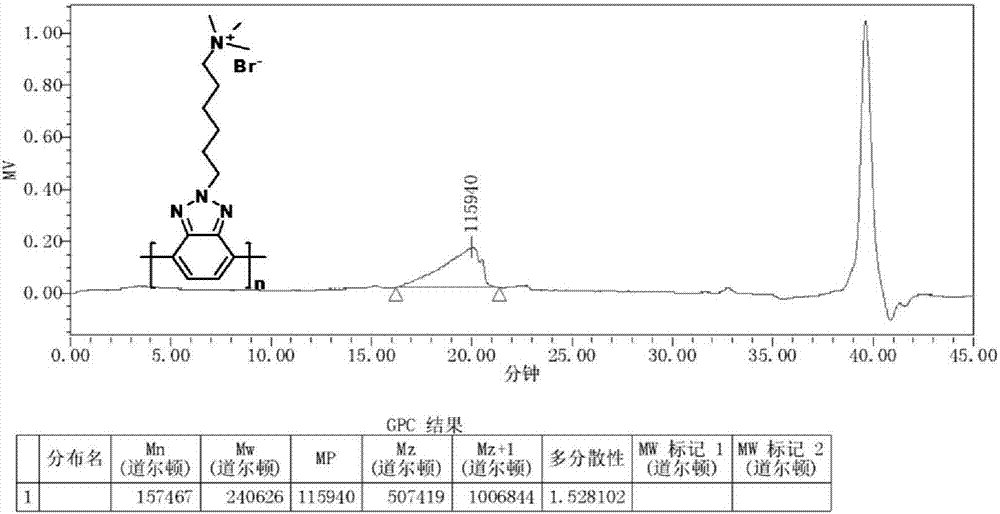
Image
Examples
Embodiment 1
[0029] Example 1: Synthesis of cationic conjugated polyelectrolytes: PBTBTz-TMABr and PBTBTz-PyrBr using Suzuki coupling polymerization method.
[0030] The synthesis process of PBTBTz-TMABr and PBTBTz-PyrBr is as follows:
[0031]
[0032] reaction process:
[0033] (i) 4,7-dibromo-2H-benzotriazole, 1,4-dibromobutyl and potassium hydroxide were reacted at 60°C for 5h.
[0034] (ii) Tetrakis(triphenylphosphine)palladium, sodium carbonate, 4,7-dibromo-2-(6-bromo-hexyl)-2H-benzotriazole and 4,7-bis-(4,4, 5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-benzo[1,2,5]thiadiazole in THF / H 2 In O solvent, react at 90°C for 24h.
[0035] (iii) The side chain copolymer containing bromine at the end is placed in THF / H 2 O solvent, add trimethylamine or pyridine, react at 60°C for 24 hours.
[0036] The synthesis process of the conjugated polyelectrolyte is: under the nitrogen atmosphere of the glove box, add the component 2 (1.0 molar ratio), namely 4,7-bis-(4,4,5,5-tetramethyl -[1,3,...
Embodiment 2
[0037] Example 2: Synthesis of PBTBTz-SO using Suzuki coupling polymerization method 3 Na
[0038] PBTBTz-SO 3 The Na synthesis process is as follows:
[0039]
[0040]The synthesis process of the conjugated polyelectrolyte is: under the nitrogen atmosphere of the glove box, add component 3 (1.0 mole) respectively, namely 4,7-dibromo-2-(4-sodium sulfonate-butyl) in the microwave tube -2H-Benzotriazole, component 4 (1.0 mol), 4,7-bis-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolane -2-yl)-benzo[1,2,5]thiadiazole, sodium carbonate (5.0 mol) and catalyst tetrakis(triphenylphosphine) palladium (2% mol) and a stirring bar, and then the microwave tube Seal and take out the glove box. The mixed solvent of dimethylformamide and water (volume ratio 4 / 1) was degassed and transferred directly into a microwave tube under a nitrogen atmosphere. The solution was placed in an oil bath, stirred at 80°C for 24 hours, and then poured into acetone to produce a red precipitate. The red precipit...
Embodiment 3
[0041] Example 3: Synthesis of PBTz-PyrBr, PBTz-TMABr and PBTz-SO using Yamamoto polymerization method 3 Na
[0042] In order to further improve the solubility of polyelectrolytes based on benzotriazole and benzothiadiazole backbones, homopolyelectrolytes with long side chains, that is, homopolybenzotriazoles with ionic side chains, are the most ideal choice. The synthesis process is as follows:
[0043]
[0044] Reaction conditions:
[0045] (i) 4,7-dibromo-2H-benzotriazole, 1,4-dibromobutane and potassium hydroxide were refluxed at 60°C for 5h.
[0046] (ii) Tetrakis(triphenylphosphine) palladium, sodium carbonate, 4,7-dibromo-2-(6-bromo-hexyl)-2H-benzotriazole in toluene / H 2 O solvent at 90°C for 24h.
[0047] (iii) The side chain homopolymer containing bromine at the end is placed in THF / H 2 O solvent, add trimethylamine or pyridine, react at 60°C for 24 hours.
[0048] (iv) 4,7-dibromo-2-(6-bromo-hexyl)-2H-benzotriazole 4,7-dibromo-2-(6-bromo-hexyl)-2H-benzotriaz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com