Non-conjugated fluorescent polymer as well as preparation method and application thereof
A fluorescent polymer and non-conjugated technology, applied in the field of fluorescent materials, can solve the problems of polymer luminescent properties not being significantly improved, easy cross-linking, low efficiency, etc., to achieve excellent photobleaching resistance, diverse and controllable structures, Excellent bonding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
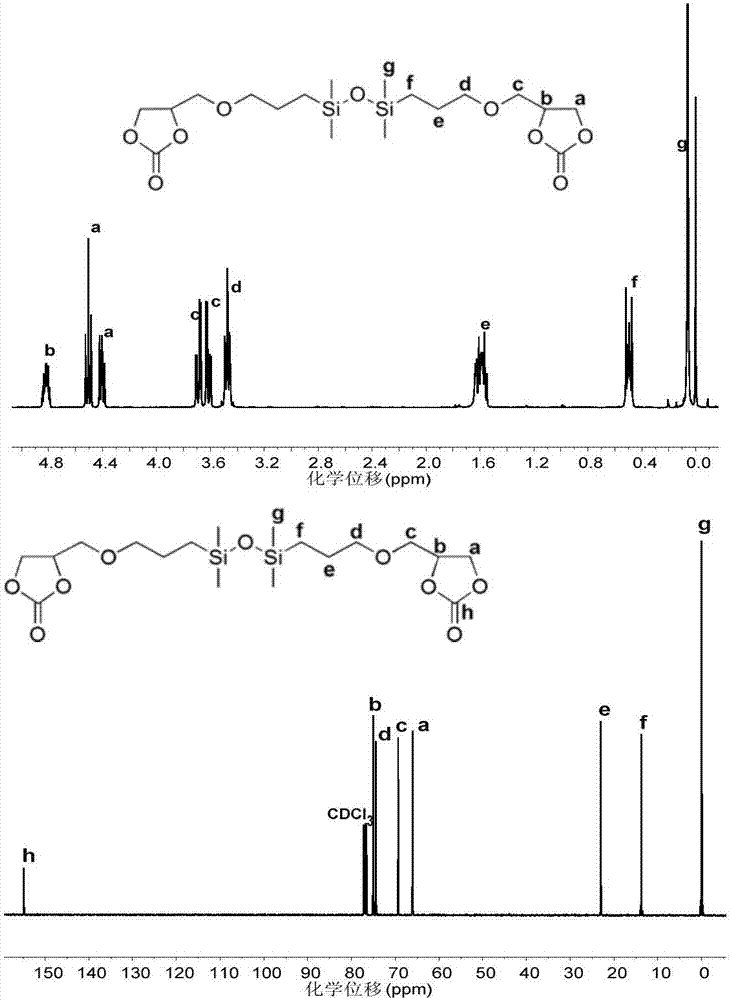
[0057] (1) First mix 50mL 1,3-bis(3-glycidyloxypropyl)tetramethyldisiloxane (CAS No: 126-80-7), 1.177g hexadecyltrimethylbromide Ammonium chloride (CAS No: 57-09-0) and 40 mg of nanometer zinc-cobalt double metal cyanide complex [Zn-Co(III) DMCC] were added to a 100 mL autoclave, and then filled with 3 MPa of carbon dioxide gas in In the reaction kettle, heat the reaction kettle to 110°C and react for 24 hours. After cooling the reaction kettle to room temperature, the unreacted carbon dioxide is released to obtain a light yellow reaction product. Remove the catalyzer cetyltrimethylammonium bromide and zinc-cobalt double metal cyanide complex to obtain the transparent oily coupling product whose end group is five-membered cyclic carbonate, the structural formula is as follows, 1 H NMR and 13 The spectrum of C NMR is as figure 1 :
[0058]
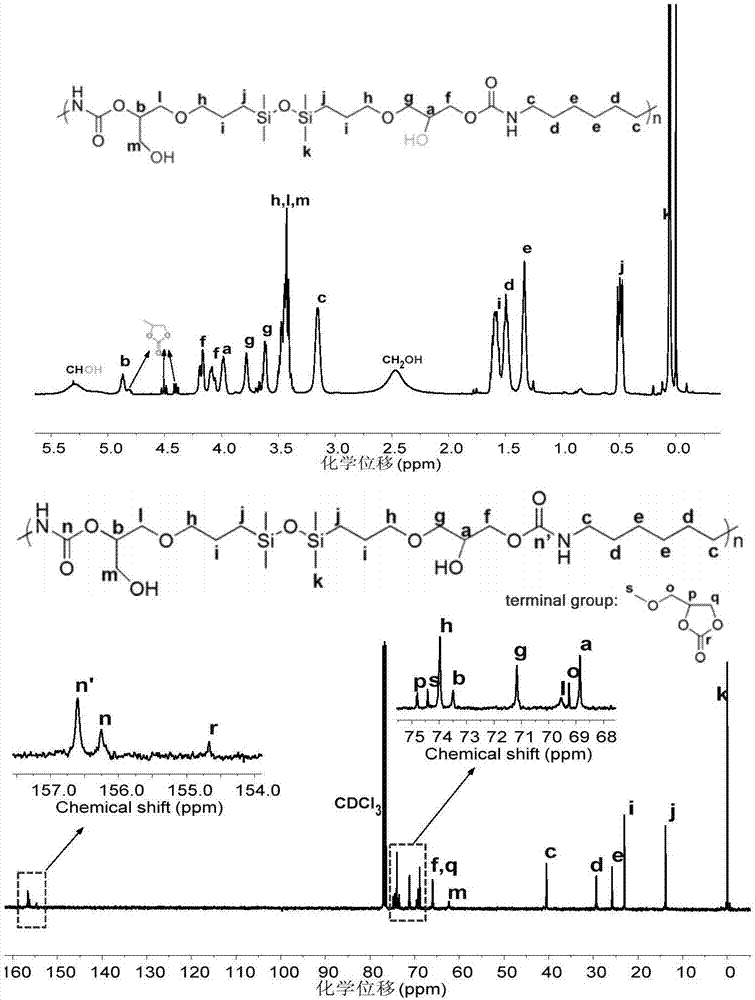
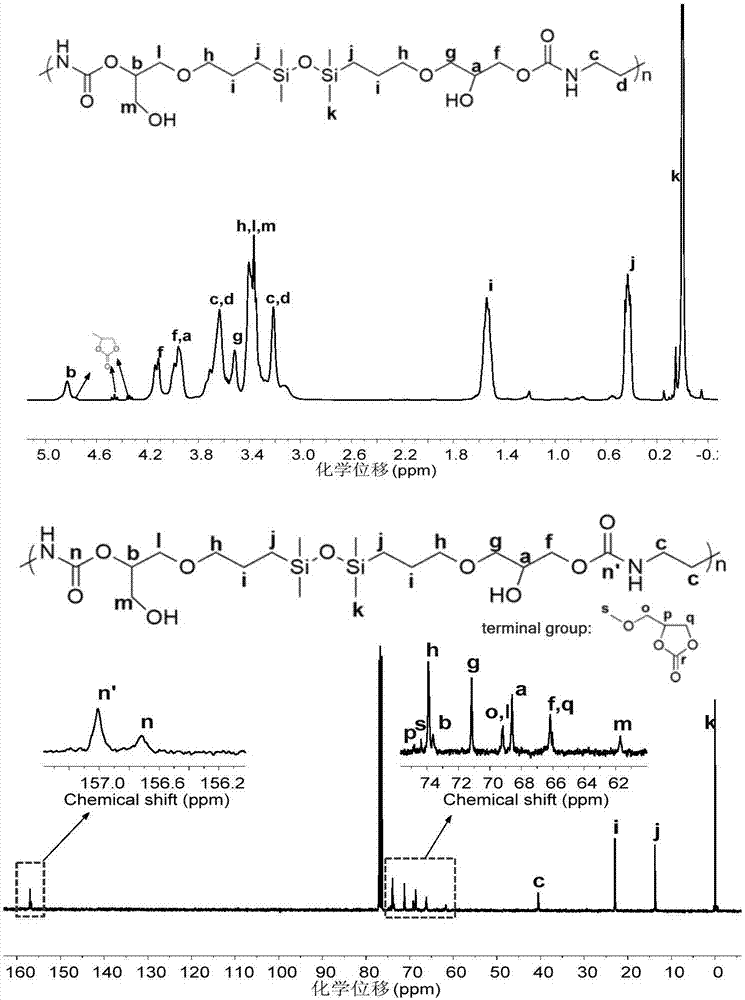
[0059] (2) Add 3 mL of the coupling product prepared in step (1) into a three-necked flask, and add another 0.9457 g of 1,6-hexameth...
Embodiment 2
[0064] (1) The preparation of the linear fluorescent polymer is the same as in Example 1.
[0065] (2) 0.01 g of the obtained linear fluorescent polymer and 0.99 g of polyethylene terephthalate are miscible in 100 mL of tetrahydrofuran, and after mixing evenly, the solvent is evaporated under reduced pressure to obtain a fluorescent polyethylene terephthalate The composition of ethylene glycol diformate and non-isocyanate polyurethane can significantly improve the strength and high temperature resistance of the material, and can be used in the fields of fluorescent fibers or fluorescent films, and its tetrahydrofuran solution can also fluoresce, which has obvious excitation dependence sex.
Embodiment 3
[0067] (1) The preparation of the linear fluorescent polymer is the same as in Example 1.
[0068] (2) 0.50 g of the obtained linear fluorescent polymer and 0.50 g of polyethylene terephthalate are miscible in 100 mL of tetrahydrofuran, and after mixing evenly, the solvent is evaporated under reduced pressure to obtain a fluorescent polyethylene terephthalate The composition of ethylene glycol diformate and non-isocyanate polyurethane can significantly improve the strength and high temperature resistance of the material, and can be used in the fields of fluorescent fibers or fluorescent films, and its tetrahydrofuran solution can also fluoresce, which has obvious excitation dependence sex.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com