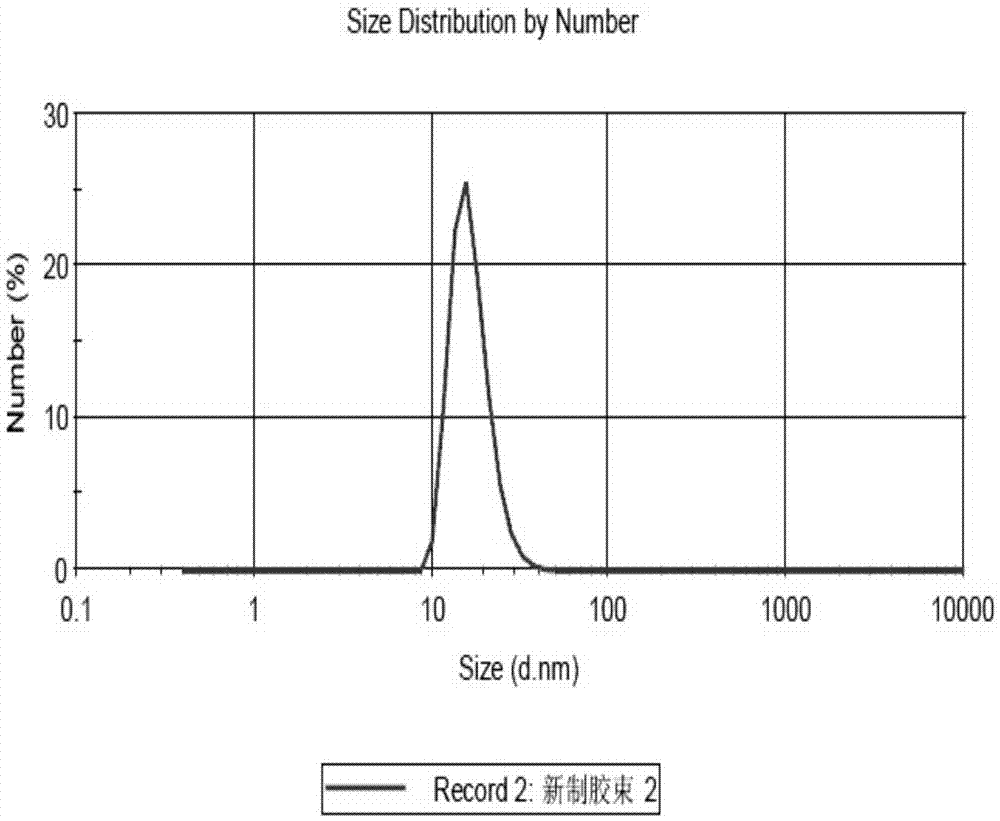
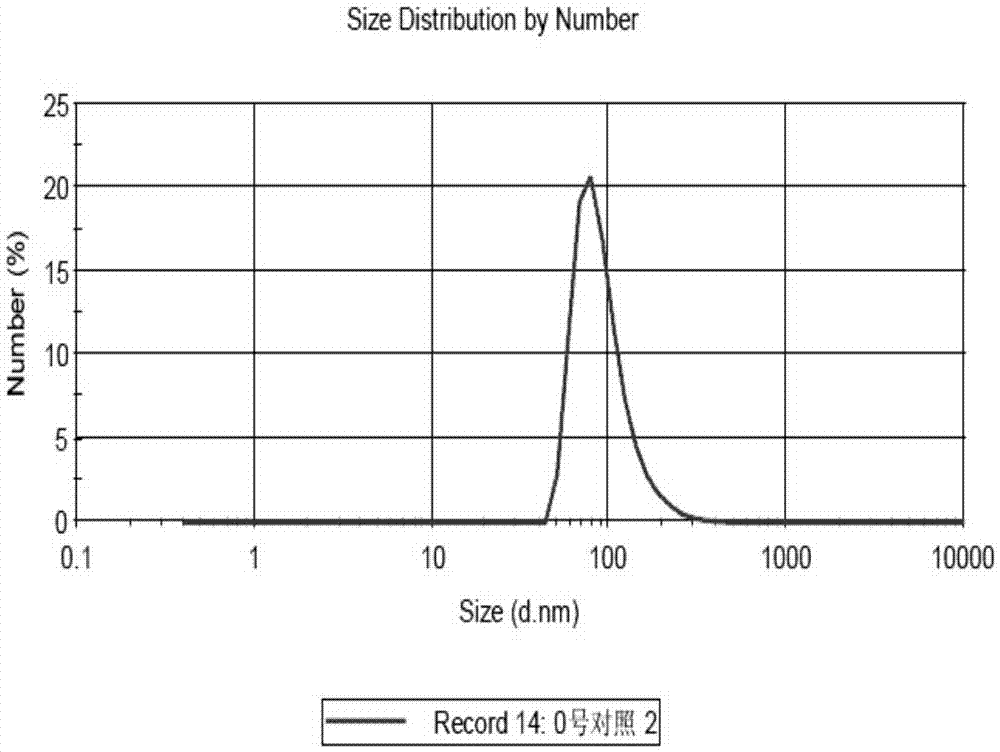
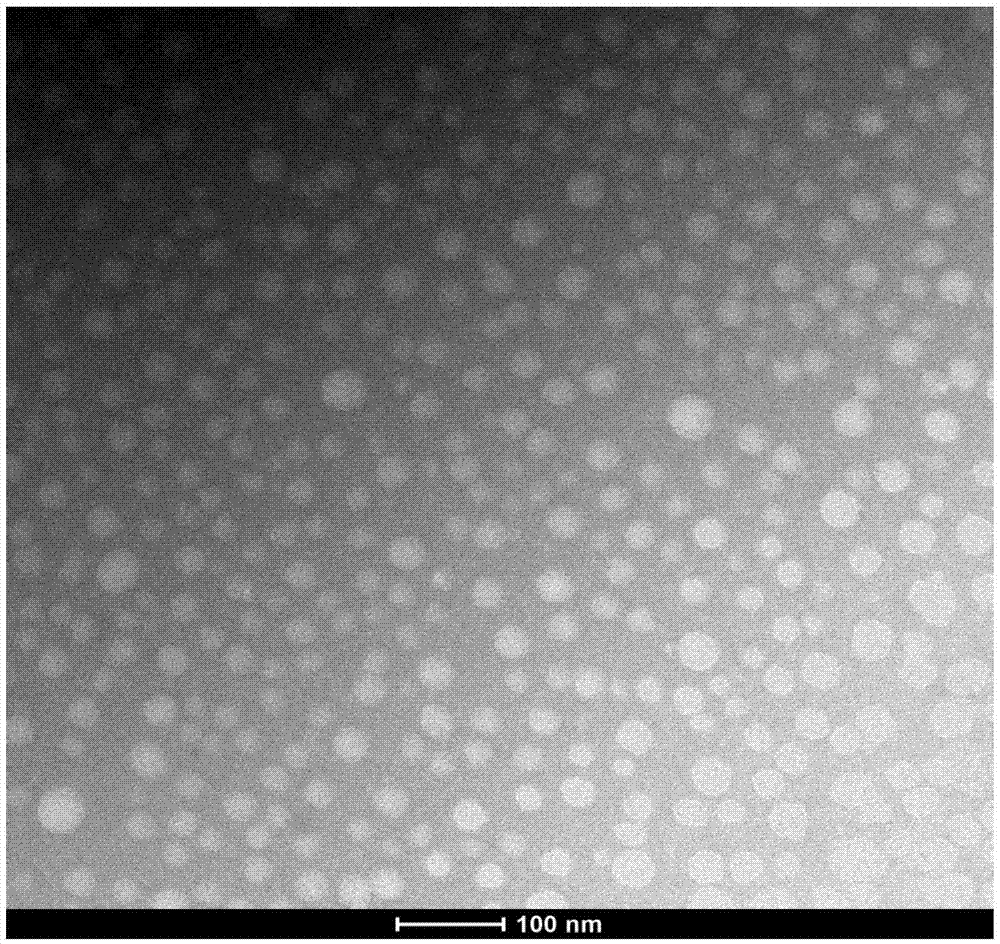
PM (PTXPEG-PLA micelles)-coated nano-cluster and preparation method thereof
A kind of micellar nano-paclitaxel technology, which is applied in the direction of pharmaceutical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of no increase in drug accumulation in tumor sites, shortened circulation time in the body, and nanoparticle particle size. In order to achieve the effect of reducing adverse reactions, increasing relative accumulation, and improving therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Weigh 50 mg PEG-PLA and 10 mg paclitaxel, add 4 ml acetonitrile, and ultrasonically dissolve the carrier material and drug fully. Place the solution in a rotary evaporator at 40°C for 30 minutes to evaporate the organic solvent to dryness, and place it in a vacuum oven overnight at room temperature to remove a small amount of residual solvent to obtain a dry and transparent drug film skeleton, then add 10ml of deionized water in the Stir at a constant speed of 500 rpm for 30 min, pass the hydration solution through a 0.22 μm cellulose acetate filter membrane to obtain a paclitaxel PEG-PLA micellar solution, add 0.5% PEI 200 μl to the paclitaxel PEG-PLA micellar solution under stirring at 500 rpm, Then 1ml of dichloromethane solution containing 20mgTPGS-CDI was added dropwise, the resulting emulsion was emulsified and ultrasonicated for 30min, and the organic solvent was removed by rotary evaporation at 40°C, 1mg IgG was added, and incubated at room temperature for 1h to ...
Embodiment 2
[0058] Weigh 70mg of PEG-PLA and 10mg of paclitaxel, add 4ml of dichloromethane, ultrasonically dissolve the carrier material and the drug, place the solution in a rotary evaporator at 40°C for 30min, and evaporate the organic solvent to dryness thoroughly. Put it in a vacuum drying oven overnight, remove a small amount of residual solvent to obtain a dry and transparent drug film skeleton, then add 10ml of normal saline and stir at a constant speed of 500rpm for 30min. Pass the hydration solution through a 0.22 μm cellulose acetate filter membrane to obtain a paclitaxel PEG-PLA micellar solution, add 200 μl of 0.5% PEI to the paclitaxel PEG-PLA micellar solution under stirring at 500 rpm, and add dropwise 1 ml containing 20 mg TPGS-CDI The resulting emulsion was emulsified and ultrasonicated for 30 min, and the organic solvent was removed by rotary evaporation at 30°C, 1 mg IgG was added, and incubated at room temperature for 1 h to prepare paclitaxel-encapsulated micellar nan...
Embodiment 3
[0060] Weigh 100mg PEG-PLA, 30mg paclitaxel, add 4ml dichloromethane, ultrasonically dissolve the carrier material and the drug, place the solution in a rotary evaporator at 50°C for 30min to evaporate the organic solvent thoroughly, and place in room temperature Put it in a vacuum drying oven overnight, remove a small amount of residual solvent to obtain a dry and transparent drug film skeleton, then add 15ml of normal saline and stir at a constant speed of 500rpm for 30min. Pass the hydration solution through a 0.22 μm cellulose acetate filter membrane to obtain a paclitaxel PEG-PLA micellar solution, add 0.5% PEI 300 μl to the paclitaxel PEG-PLA micellar solution under stirring at 500 rpm, and add dropwise 1 ml containing 20 mg PEG-CDI The resulting emulsion was emulsified and ultrasonicated for 30 min, and the organic solvent was removed by rotary evaporation at 40 °C, 1.5 mg of IgG was added, and incubated at room temperature for 1 h to prepare paclitaxel-encapsulated mice...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com