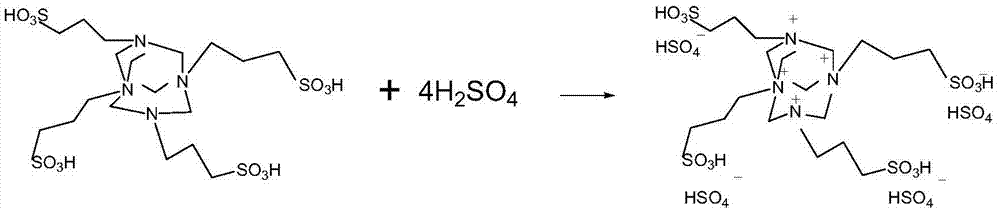Method for preparing raspberry ketone under catalysis of multi-sulfonic functionalized ionic liquid
A functional ionic liquid and polysulfonic acid-based technology is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds. It can solve problems such as limiting industrial application value, easy to pollute the environment, and harsh reaction conditions. Improve the industrial application value, reduce the reaction temperature, and improve the effect of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
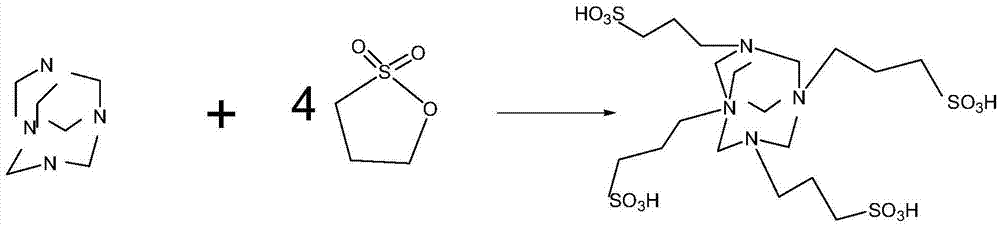
[0026] (1) Preparation of catalyst: 145g of hexamethylenetetramine and 368g of 1,3-propane sultone were mixed and stirred at room temperature for 6 hours, and 500ml of ethyl acetate was added as a solvent; Washed three times with ethyl acetate, and dried in vacuum at 60°C to obtain 480 g of ylide, with a yield of 95% relative to 1,3-propane sultone;
[0027] (2) Preparation of raspberry ketone: 480 g of ylide and 400 g of concentrated sulfuric acid were weighed and stirred at 80° C. for 4 hours to obtain the corresponding ionic liquid. 0.6 mole of phenol, 0.8 mole of butenone and 0.2 mole of ionic liquid were put into the reaction vessel, and stirred at 30° C. for 8 hours. Add 2 moles of water, filter and separate to obtain raspberry ketone, and the reaction yield is 79%; unreacted raw materials and catalysts can be used next time after dehydration. The reaction formula is as follows:
[0028]
Embodiment 2~4
[0030] The addition reaction temperature of step (2) of Examples 2 to 4 is shown in Table 1, and other reaction conditions are the same as in Example 1, and the reaction results are shown in Table 1.
[0031] The reaction condition and reaction result of table 1 embodiment 2~4
[0032] Example Reaction temperature °C Yield% 2 40 91 3 50 93 4 60 74
Embodiment 5~7
[0034] The operation of Examples 5-7 is basically the same as that of Example 2, except that the step (2) does not use an ionic liquid as a catalyst, but directly uses an acid to catalyze the reaction. The acid used and the reaction results are shown in Table 2.
[0035] Example acid Yield% 5 sulfuric acid 72 6 phosphoric acid 51 7 p-Toluenesulfonic acid 38
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com