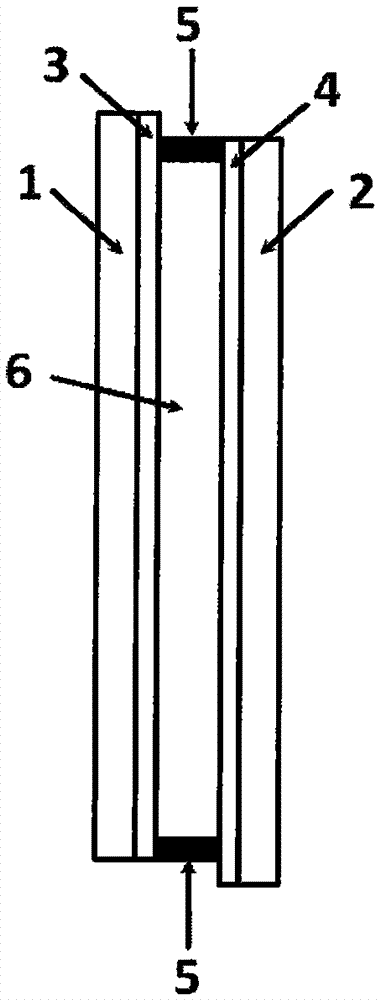
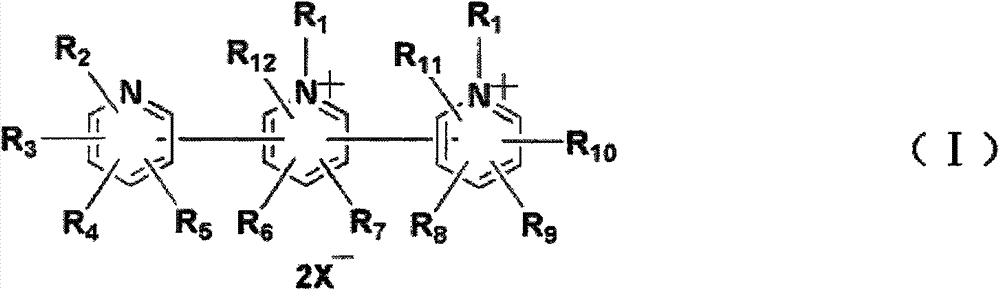
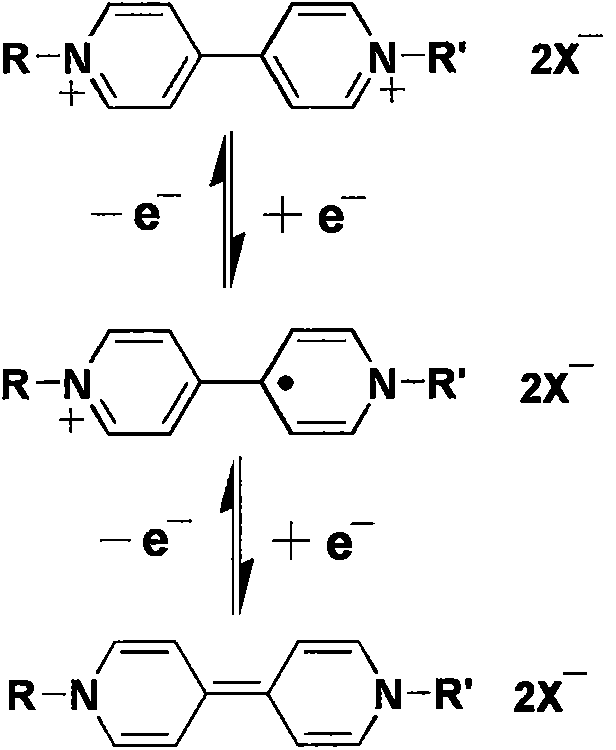Cathodic electrochromic compound used in electrochromic device, and preparation method thereof
An electrochromic and compound technology, applied in chemical instruments and methods, color-changing fluorescent materials, instruments, etc., can solve the problems of increasing the extinction coefficient and achieve the effects of increasing the extinction coefficient, excellent resistance to ultraviolet radiation, and reducing light transmittance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) synthesize the cathodic electrochromic compound of following structural formula:
[0046]
[0047] The synthetic route and operation steps are as follows:
[0048]
[0049] Step 1: Disperse sodium hydroxide (20mmol) in polyethylene glycol (200mL) with an average molecular weight of 300g / mol, then add 4-acetylpyridine (20mmol) and benzaldehyde (10mmol) sequentially at 0°C , and react at 0°C for 2h;
[0050] The second step: add ammonium acetate (0.5mol) to the reaction system obtained in the first step, and then incubate at 100° C. for 2 hours, during which precipitation gradually occurs;
[0051] The third step: add deionized water (500 mL) to the reaction system in the second step, and filter to obtain a precipitate. The obtained precipitate was washed with water and a small amount of ethanol in sequence, and dried under vacuum at 50°C for 12 hours to obtain intermediate 4;
[0052] Step 4: Take the intermediate product 4 (4 mmol) and dissolve it in ethano...
Embodiment 2
[0068] (1) synthesize the cathodic electrochromic compound of following structural formula:
[0069]
[0070] The synthetic route and operation steps are as follows:
[0071]
[0072] The first step: under the protection of nitrogen, a n-hexane solution of triethylborane (concentration is 1mol / L, volume is 21mL) is added dropwise to the solution containing 3-lithium pyridine (by butyllithium (21mmol) and 3-bromo Pyridine (21mmol) was prepared by reaction in n-hexane at -5-0°C) in ether solution at -78°C, after the dropwise addition was completed, the reaction was continued at -78°C for 1 h and at room temperature for one night. After the reaction was completed, a solution of iodine (21 mmol) in tetrahydrofuran (20 mL) was added and stirred for 1 h. Afterwards, the above solution was diluted with ethyl acetate and treated with 10% Na 2 S 2 o 3 It was washed with aqueous solution, and the organic phase was collected by static layering and dried with magnesium sulfate. ...
Embodiment 3
[0090] (1) synthesize the cathodic electrochromic compound of following structural formula:
[0091]
[0092] The synthetic route and operation steps are as follows:
[0093]
[0094] The first step: under the protection of nitrogen, a n-hexane solution of triethylborane (concentration is 1mol / L, volume is 21mL) is added dropwise to the solution containing 4-lithium pyridine (by butyllithium (21mmol) and 4-bromo Pyridine (21mmol) was prepared by reaction in n-hexane at -5-0°C) in ether solution at -78°C. After the dropwise addition was completed, the reaction was continued at -78°C for 1 h and at room temperature for one night. After the reaction was completed, a solution of iodine (21 mmol) in tetrahydrofuran (20 mL) was added and stirred for 1 h. Afterwards, the above solution was diluted with ethyl acetate and treated with 10% Na 2 S 2 o 3 It was washed with aqueous solution, and the organic phase was collected by static layering and dried with magnesium sulfate. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com