A kind of preparation technology of Lansoprazole
A technology for the preparation of lansoprazole and a preparation process, which is applied in the field of preparation of pharmaceutical compounds, can solve the problems of backward raw material preparation process, uneasy control of the crystallization process, unstable product performance, etc. It is simple and easy to meet equipment requirements, shorten the production cycle, The effect of material stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
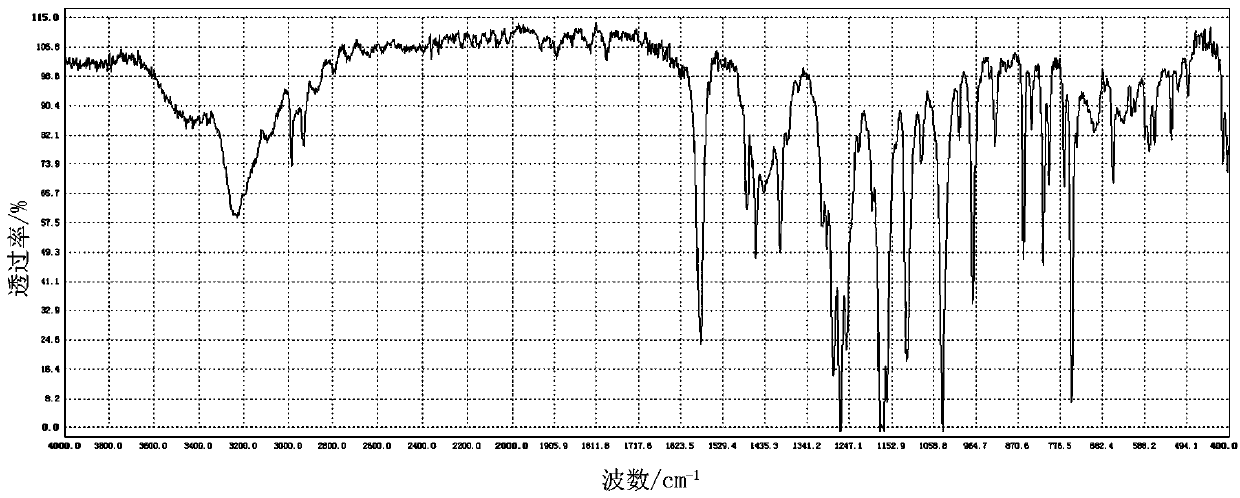
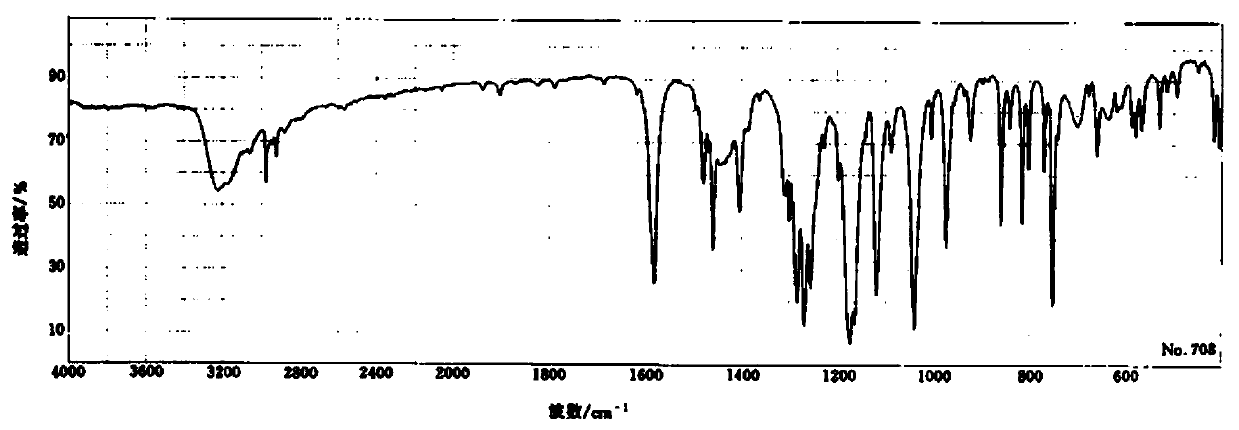
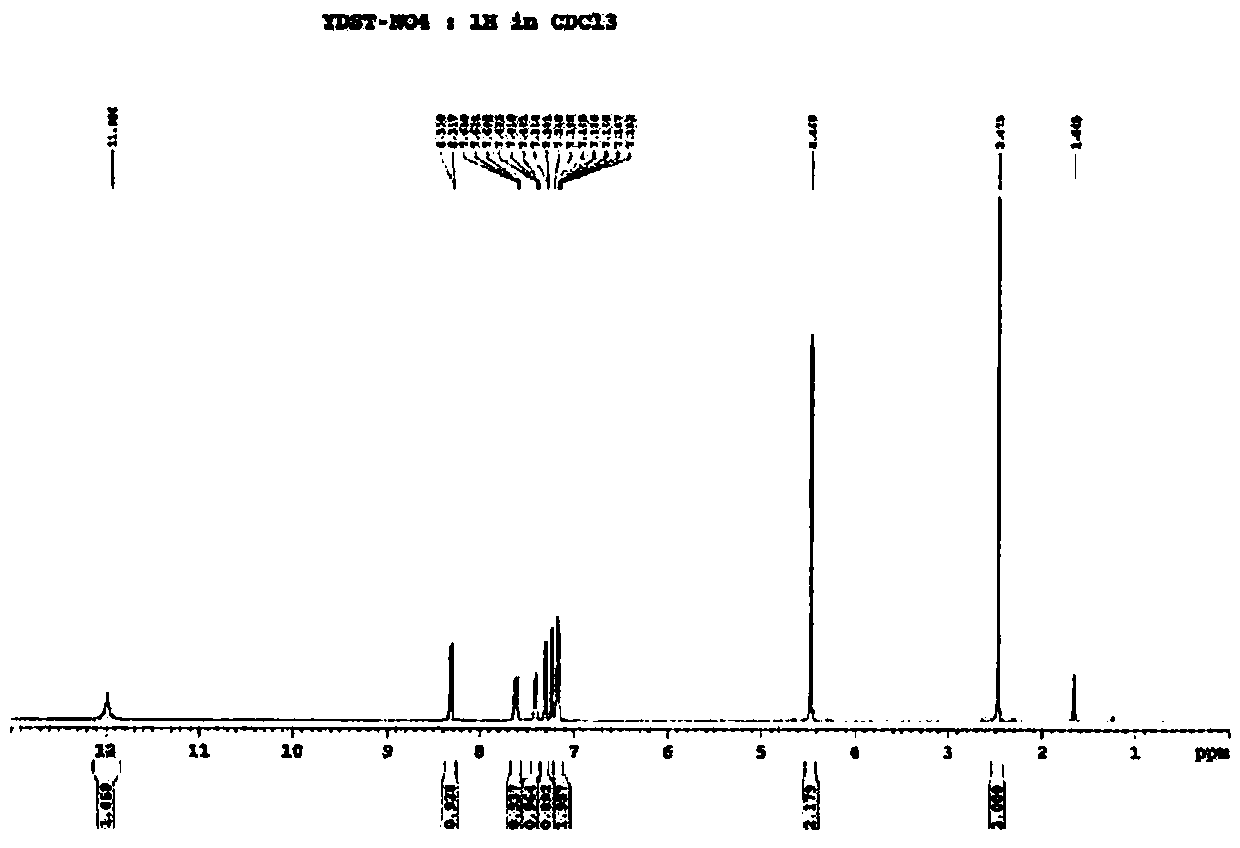
Image
Examples
Embodiment 1
[0031] A preparation technique for Lansoprazole, comprising the following steps:
[0032] (a) In a clean glass kettle, first add 6L of anhydrous methanol, then add 0.7kg of 2-mercaptobenzimidazole (raw material A) and 0.5kg of solid sodium hydroxide under stirring, raise the temperature to 40-50°C, and control the temperature. After stirring and dissolving, add 1kg of 2-chloromethyl-3-methyl-4-(2,2,2,-trifluoroethoxy)pyridine hydrochloride (raw material B) to dissolve, react for 2 hours, add appropriate amount of purification water, cool down to room temperature, spin filter, wash the precipitate with purified water, spin dry, and vacuum dry at 45°C to obtain intermediate C;
[0033] (b) Add intermediate C to 10L of 95% ethanol, control the temperature at 10-15°C, stir and dissolve, then add a mixture of 0.6kg of 30% hydrogen peroxide, 0.02kg of catalyst and 0.3 kg of ethanol, and react for 2 hours under temperature control. After HPLC detection, the residue of intermediate C...
Embodiment 2
[0036] A preparation technique for Lansoprazole, comprising the following steps:
[0037] (a) In a clean glass kettle, first add 7L of anhydrous methanol, then add 0.8kg of 2-mercaptobenzimidazole (raw material A) and 0.6kg of solid sodium hydroxide under stirring, raise the temperature to 40-50°C, and control the temperature. After stirring and dissolving, add 1kg of 2-chloromethyl-3-methyl-4-(2,2,2,-trifluoroethoxy)pyridine hydrochloride (raw material B) to dissolve, react for 2 hours, add appropriate amount of purification water, cool down to room temperature, spin filter, wash the precipitate with purified water, spin dry, and vacuum dry at 45°C to obtain intermediate C.
[0038] (b) In a clean glass kettle, first add 10L of 95% ethanol, then add the intermediate C obtained above under stirring, control the temperature at 10-15°C, stir and dissolve, then add 0.6kg of 30% hydrogen peroxide and 0.05kg of catalyst The mixed solution mixed with 0.48 kg ethanol was reacted for...
Embodiment 3
[0041] A preparation technique for Lansoprazole, comprising the following steps:
[0042] (a) In a clean glass kettle, first add 8L of anhydrous methanol, then add 0.8kg of 2-mercaptobenzimidazole (raw material A) and 0.6kg of solid sodium hydroxide under stirring, raise the temperature to 40-50°C, control the temperature, and stir After dissolving, add 1 kg of 2-chloromethyl-3-methyl-4-(2,2,2,-trifluoroethoxy)pyridine hydrochloride (raw material B) to dissolve, react for 2 hours, add appropriate amount of purified water, Cool down to room temperature, spin filter, wash with purified water, spin dry, and vacuum dry at 45°C to obtain intermediate C.
[0043] (b) In a clean glass kettle, first add 10L of 95% ethanol, then add the intermediate C obtained above under stirring, control the temperature at 10-15°C, stir and dissolve, then add 0.3kg of 30% hydrogen peroxide and 0.05kg of catalyst The mixed solution mixed with 0.24 kg ethanol was reacted for 2 hours under temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com