Preparation method for novel antifungal compound and antifungal application
A compound, antifungal technology, applied in the field of medicine, can solve the problems of human toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
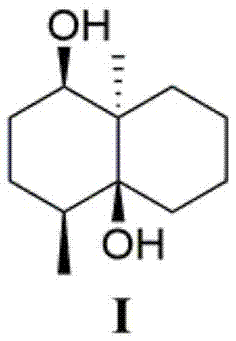
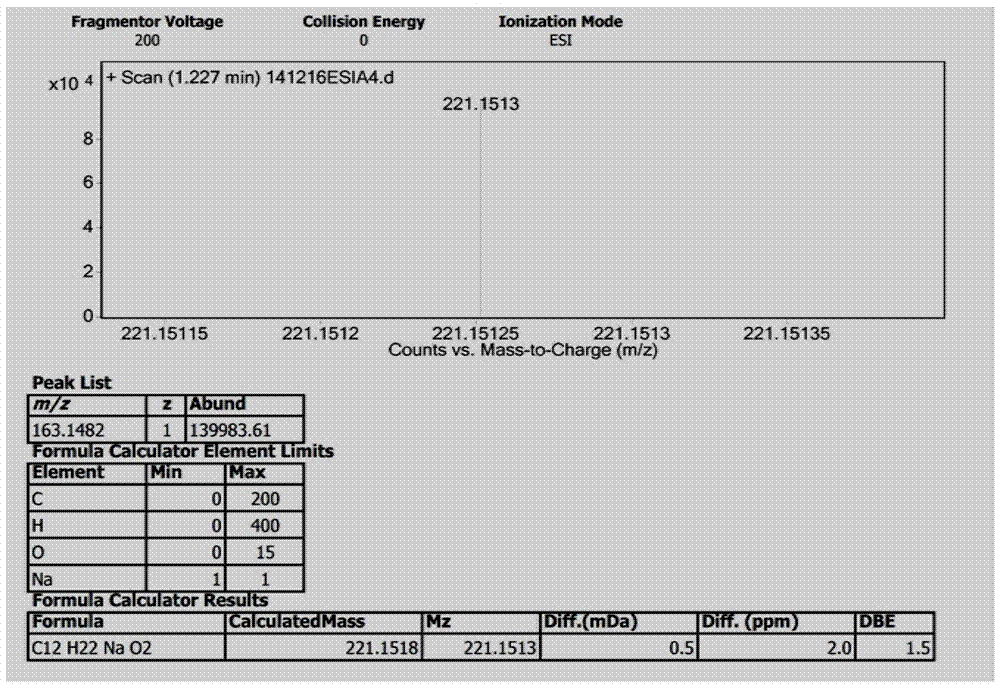
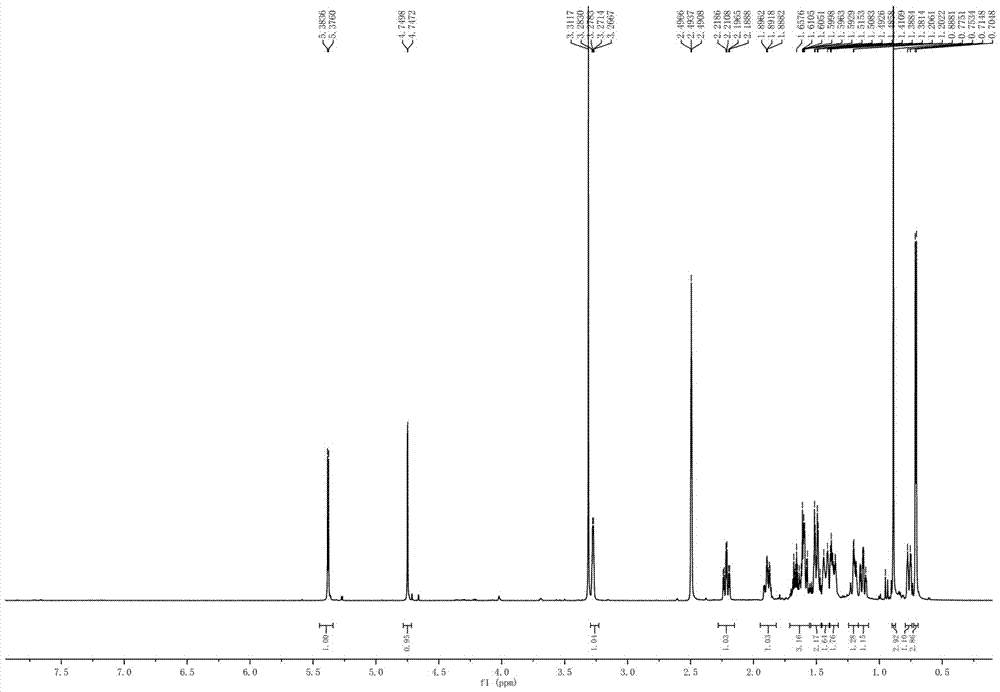
[0036] Preparation of new compound I
[0037] 1.1 Seed culture of Streptomyces albolongus.
[0038] Seed medium: 0.4 g of yeast extract, 0.4 g of glucose, 0.5 g of malt extract, 0.1 mL of multivitamins, 1.0 mL of trace elements, and 100 mL of distilled water. Culture conditions: pH 7.2, culture temperature 28 degrees Celsius, culture time 48 hours.
[0039] 1.2 Fermentation of Streptomyces albolongus.
[0040] Fermentation medium: soybean powder 10 g / L, peptone 2 g / L, glucose 20 g / L, soluble starch 5 g / L, yeast extract 2 g / L, sodium chloride 4 g / L, dipotassium hydrogen phosphate 0.5 g / L, magnesium sulfate 0.5 g / L, calcium carbonate 2 g / L, distilled water 70 L. Culture conditions: pH 7.8, culture temperature 28 degrees Celsius, culture time 168 hours.
[0041] 1.3 Extraction.
[0042] The fermentation broth obtained in step (2) was centrifuged, and the supernatant was subjected to column chromatography on XAD-16 macroporous adsorption resin (the volume of the macroporous a...
Embodiment 2
[0051] Embodiment 2: antifungal activity test
[0052] Compound I was dissolved in DMSO, and serially diluted to 9 different concentrations (22.0, 11.0, 5.5, 2.75, 1.38, 0.69, 0.36, 0.18, 0.09 mg / mL). Pathogen conditional pathogenic fungi Candida albicans ATCC MYA-2876, Candida parapsilosis ATCC 22019 and Cryptococcus neoformans ATCC 208821 were inoculated in RPMI-1640 medium (10.4 g L -1 RPMI-1640, 2 g L -1 NaHCO 3 , 34.53 g L -1 MOPs, pH 7.0), the concentration of Candida albicans (C.albicans) and Candida parapsilosis (C. parapsilosis) was 2 × 10 3 cfu / mL, the concentration of Cryptococcus neoformans (C.neoformans) bacterial suspension is 5 × 10 4 cfu / mL. The sample was diluted 10 times with RPMI-1640 medium, and 10 μL of sample and 100 μL of bacterial suspension were added to a 96-well plate. An equal volume of DMSO solution was used as a negative control, and amphotericin B was used as a positive control. The results showed that compound I showed strong inhibitory ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com