Anti-IDH1 R132H antibody as well as preparation method and application thereof
A technology of IDH1R132H and antibodies, which is applied in the fields of botany equipment and methods, biochemical equipment and methods, antibodies, etc., and can solve the problems such as the emergence of monoclonal antibodies without antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1. Production of anti-IDH1 R132H antibody
[0059] Antigen preparation
[0060] A short peptide containing IDH1 R132H (synthesized by Shanghai Qiangyao Biotechnology Co., Ltd.) was artificially synthesized and coupled with a carrier to immunize mice.
[0061] Hybridoma preparation
[0062] We used standard in vivo immunization and fusion methods to generate anti-IDH1 R132H antibodies. The brief process of this method is as follows:
[0063] Mice were immunized with IDH1 R132H antigen obtained above, and one type of antigen was mixed and emulsified with Freund's complete adjuvant in an equal volume, and injected into muscles of limbs at multiple points, each with 200 microliters per injection. After the first immunization, booster immunization was carried out with the same dose of any other type plus Freund's incomplete adjuvant. After the fourth booster immunization, blood was collected to detect the titer of reaction with it. When the titer reaches 10 6 In the ab...
Embodiment 2
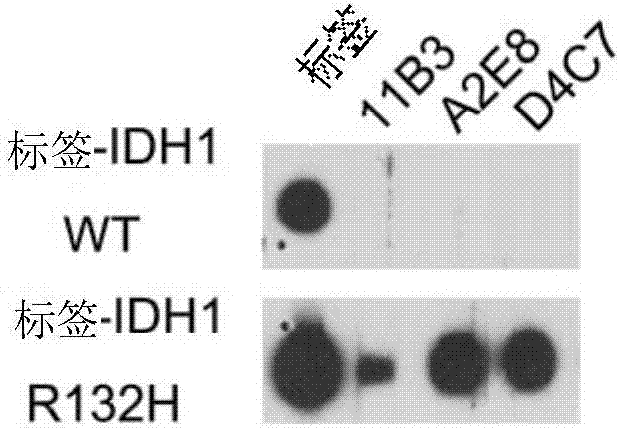
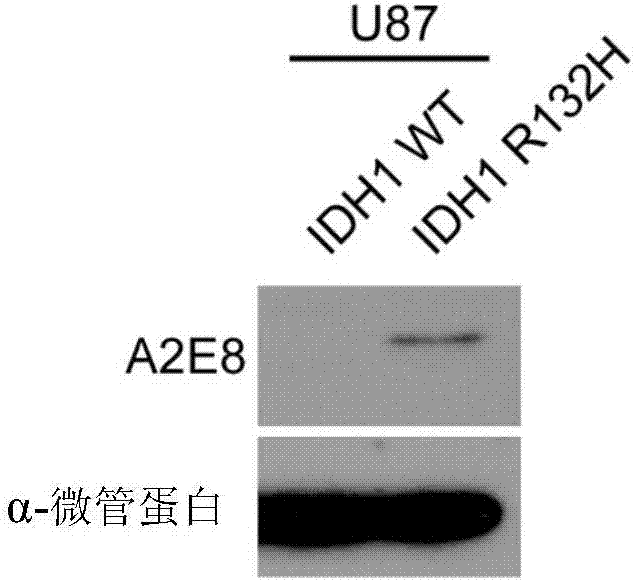
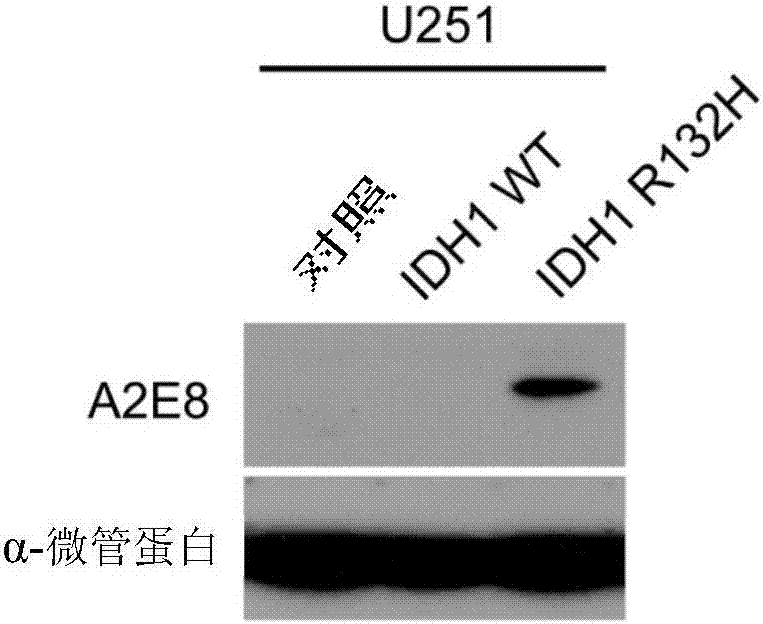
[0071] Detection of specificity of anti-IDH1 R132H antibody.
[0072] Dot blot verification:
[0073] HEK293FT cells were transfected with an overexpression vector expressing the full length of IDH1 with a Flag tag and an overexpression vector expressing IDH1 R132H. After 72 hours, the cells were lysed, and 30ul of the lysate was spotted on the NC membrane, and air-dried at room temperature. Blocked with PBS at room temperature for 1 hr, added TBST 1:5000 diluted monoclonal antibody, reacted at room temperature for 1 hr, rinsed the membrane with TBST for 10 min×3; added diluted secondary antibody (both 1:4000 dilution), incubated at room temperature for 1 h on a horizontal shaker. Absorb the secondary antibody, rinse the membrane with TBST, 10min×4, mix ECL (A:B=100:1 solution), fully cover the surface of the wet NC membrane, and let it stand for 1min; seal the NC membrane with plastic wrap in the dark room, and put The X-ray film was laid flat on the NC film to develop and r...
Embodiment 3
[0076] Isolation of variable region of light chain gene and heavy chain gene of monoclonal antibody
[0077]Polymerase chain reaction is used to isolate the variable region of the antibody gene, wherein VLF and VLR are downstream primers for gene amplification of the light chain variable region, and VLF and VLR are downstream primers for gene amplification of the heavy chain variable region. The template used was the total RNA of the hybridoma cell line extracted by the Trizol method. After the DNA was digested, it was reverse-transcribed into cDNA using a reverse transcription kit. Conditions: The PCR reaction conditions are: pre-denaturation at 95°C for 5 minutes; then 40 cycles of reaction at 95°C for 15s, 55°C for 30s, and 72°C for 50s. The fragment of interest was recovered, cloned into a T-vector, and then sequenced (Intravel Corporation). The sequencing sequences are compared to determine the nucleotide sequence of the antibody variable region, and then determine the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com