Monoclonal antibody against human lp-pla2 protein resistant to lipoprotein interference and its application
A monoclonal antibody and protein technology, applied in the direction of antibodies, immunoglobulins, antibody medical components, etc., can solve the problem of increasing the difficulty of developing monoclonal antibodies for diagnosis, the inability to produce monoclonal antibodies against Lp-PLA2, and the inability to accurately identify them, etc. problems, to achieve good specificity and temperature stability, good affinity and specificity, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Expression Purification and Purity Identification of Recombinant Lp-PLA2 Protein
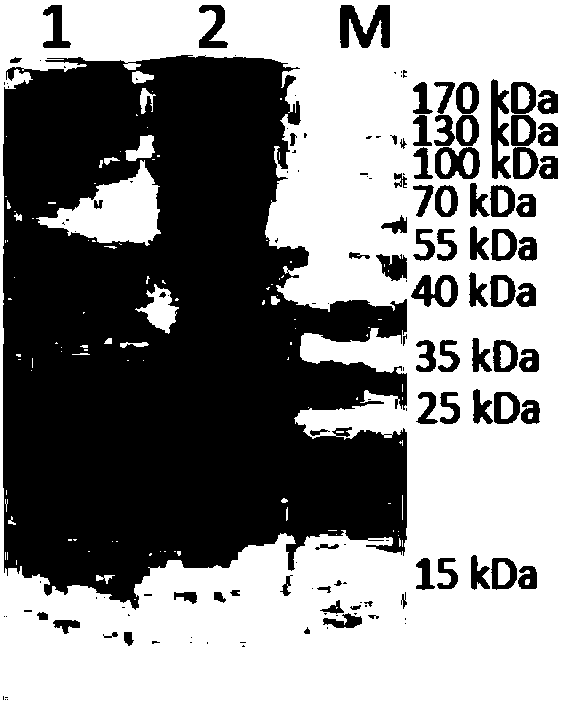
[0050] The prokaryotic expression plasmid containing the Lp-PLA2 gene sequence was transformed into Escherichia coli BL21(DE3), and cultured on LB plate. Pick a single colony from the plate and inoculate it into LB medium. After expanding the culture, when the OD600 value of the bacterial solution reaches 0.6-0.8, add IPTG with a final concentration of 0.2mM to induce expression. After 16 hours, the bacterial cells were collected by centrifugation, and the supernatant was collected by centrifugation after the bacteria were disrupted by ultrasonic. The supernatant was purified by cation exchange column and molecular sieve chromatography column, and the samples were collected and identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis for protein purity.
[0051] Carry out sodium dodecylsulfonate-polyacrylamide gel electrophoresis after purifying recombinant Lp-PL...
Embodiment 2
[0052] Example 2 Animal immunity and serum titer detection
[0053] Mix and emulsify the purified recombinant Lp-PLA2 protein with a concentration of 1 mg / ml and the same amount of complete Freund's adjuvant with double syringes, and immunize with the emulsified antigen for 6-8 weeks. Specific pathogen-free female BALB / c For mice, each mouse was injected with 40 μg antigen from the hock joint. After 2 days, the purified recombinant Lp-PLA2 protein with a concentration of 1 mg / ml and the same amount of Freund's incomplete adjuvant were mixed and emulsified with double syringes, and each mouse was injected with 40 μg emulsified Lp-PLA2 again from the hock joint. antigen. Eight days later, blood was collected from the tail vein, centrifuged to obtain the supernatant, and the serum titer was detected by indirect ELISA. After the second immunization, the titer of the serum after a million-fold dilution was as high as 2.0 or more (Table 1). Select the serum titer greater than 10 ...
Embodiment 3
[0056] Example 3 Cell Fusion, Positive Hybridoma Screening and Subcloning
[0057] 1. Preparation and screening of high-titer hybridoma cell lines
[0058] Using PEG 1500 as a fusion agent, sp2 / 0 myeloma cells and lymphocytes from immunized mice were fused, and the ratio of the two cells was 1:2-1:3. The fused cells were cultured in HAT-1640 medium containing 20% FBS serum. One week later, observe the fusion state and change the medium, and take the cell culture supernatant 3 days after the medium change. The ELISA plate was coated with the serum of patients with clinically confirmed atherosclerosis, which contains a high concentration of natural Lp-PLA2 protein, and the positive clones were screened by indirect ELISA method, and the cells with a higher ratio of positive value to cell number were selected for multiplication. After subcloning, multiple strains of hybridoma cell banks capable of stably secreting monoclonal antibodies against Lp-PLA2 protein were finally obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com