Preparation method of alpha-ethyl linolenate
A technology of ethyl linolenate and fatty acid ethyl ester, which is applied in the field of separation and preparation of chemical raw materials, can solve problems such as poor realization of industrial production, high equipment requirements, and large solvent consumption, so as to reduce the risk and purity of heavy metal residues High effect with low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) 1000g linseed oil (α-linolenic acid content 55.6%) is mixed with 400ml absolute ethanol and 40ml sodium ethylate (20% sodium ethylate ethanolic solution) to carry out transesterification, and react at a temperature of 80°C for 1 Hours, after the reaction, ethanol was recovered, and glycerin, alkali and a small amount of ethanol were separated by standing, then washed to neutrality, vacuum-dried and dehydrated to obtain mixed fatty acid ethyl esters with a α-linolenic acid ethyl ester content of 55.3%.
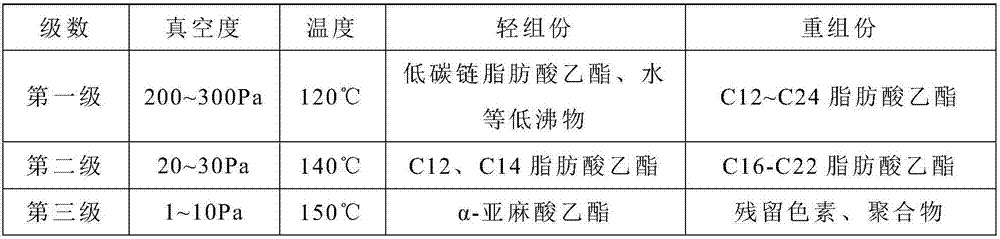
[0034] 2) The mixed fatty acid ethyl ester in step 1) is removed the first-order light component through the first-stage molecular distillation, and obtains the first-stage heavy component;
[0035] 3) removing the second-order light components from the first-level heavy components in step 2) through second-level molecular distillation to obtain the second-level heavy components;
[0036] 4) The second heavy component in step 3) was removed through the third molecular...
Embodiment 2
[0043] 1) After mixing 1000g perilla oil (α-linolenic acid content 59.9%) with 500ml absolute ethanol and 8g NaOH, carry out transesterification reaction, react at a temperature of 80°C for 2 hours, reclaim ethanol after the reaction, and statically Set aside to remove glycerin, alkali and a small amount of ethanol, then wash with water until neutral, dry and dehydrate in vacuum to obtain mixed fatty acid ethyl esters with a α-linolenic acid ethyl ester content of 59.3%.
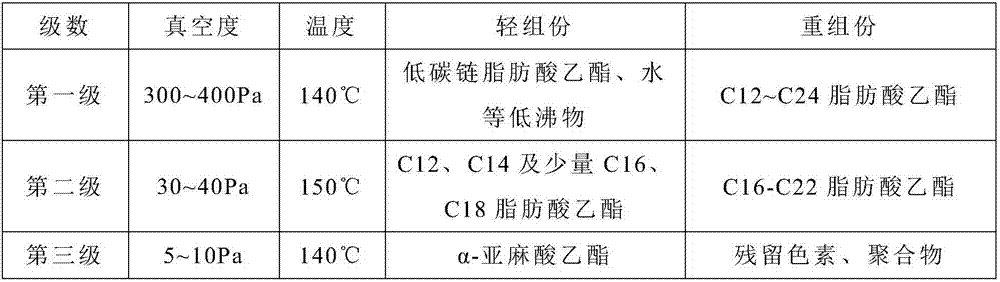
[0044] 2) The mixed fatty acid ethyl ester in step 1) is removed the first-order light component through the first-stage molecular distillation, and obtains the first-stage heavy component;
[0045] 3) removing the second-order light components from the first-level heavy components in step 2) through second-level molecular distillation to obtain the second-level heavy components;
[0046] 4) The second heavy component in step 3) was removed through the third molecular distillation to obtain 814 g of the thir...
Embodiment 3
[0053] 1) 1000g Eucommia ulmoides seed oil (α-linolenic acid content 60.3%) is mixed with 600ml absolute ethanol and 6g KOH to carry out transesterification reaction, react at a temperature of 80° C. for 2 hours, reclaim ethanol after the reaction, and statically Set aside to remove glycerin, alkali and a small amount of ethanol, then wash with water until neutral, dry and dehydrate in vacuum to obtain mixed fatty acid ethyl esters with a α-linolenic acid ethyl ester content of 59.4%.
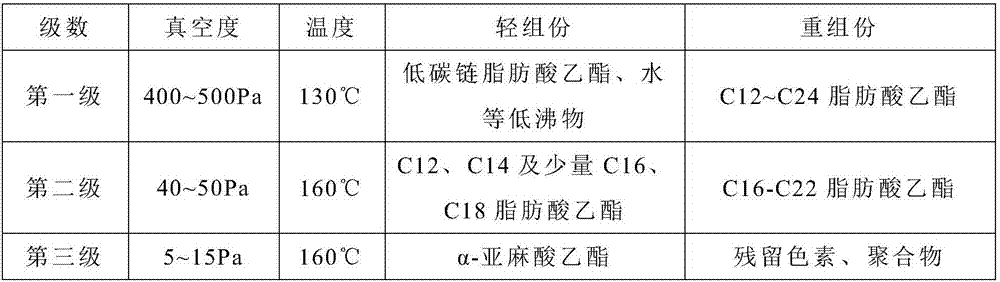
[0054] 2) The mixed fatty acid ethyl ester in step 1) is removed the first-order light component through the first-stage molecular distillation, and obtains the first-stage heavy component;
[0055] 3) removing the second-order light components from the first-level heavy components in step 2) through second-level molecular distillation to obtain the second-level heavy components;
[0056] 4) The second heavy component in step 3) was removed through the third level molecular distillation to obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com