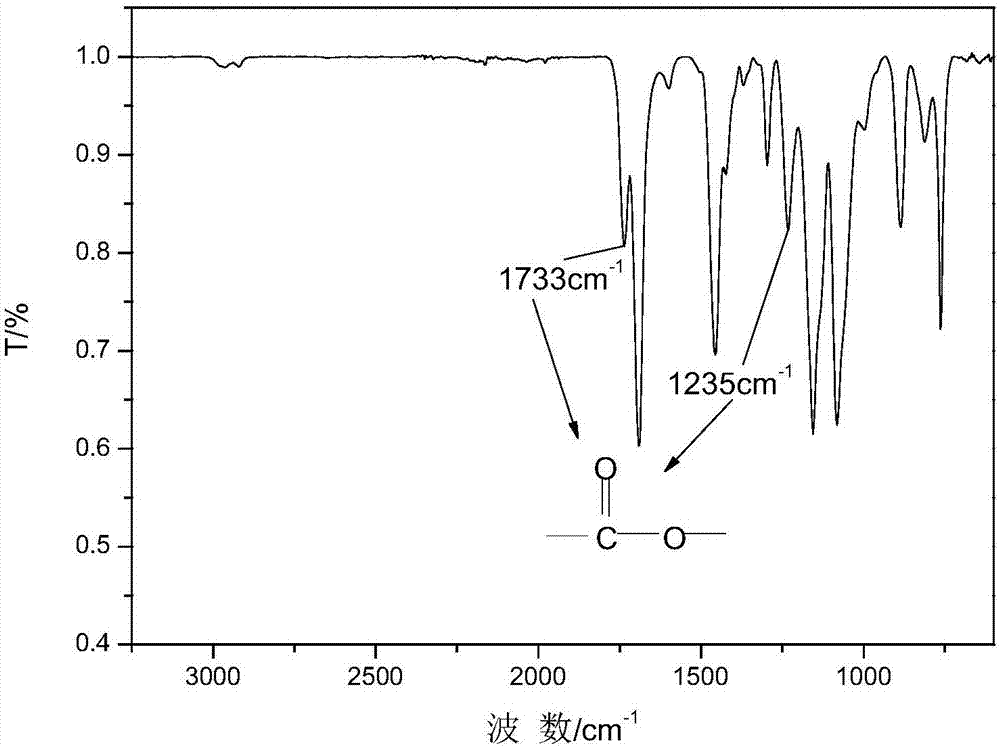
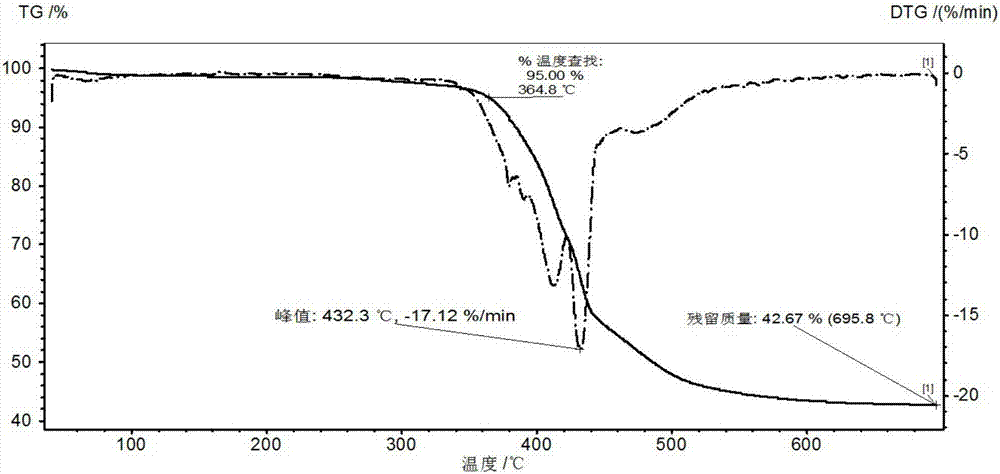
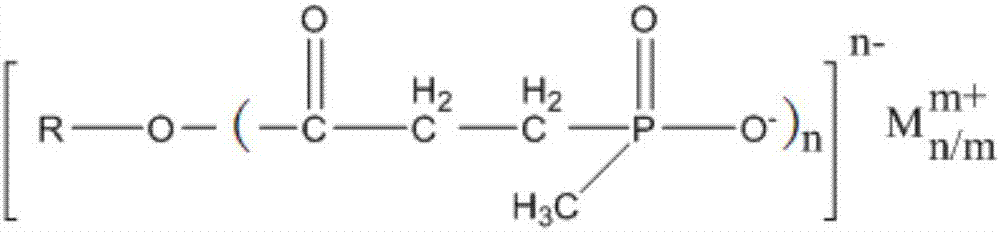
Single molecular expansion type alkyl phosphinate flame retardant and preparation method thereof
A technology of alkyl phosphinate and methyl propionate phosphinic acid is applied in the field of flame retardant materials, which can solve the problems of great influence on physical and mechanical properties of substrates, easy deliquescence and precipitation, and low thermal stability, etc. Achieve the effect of retaining mechanical properties, good anti-migration, and preventing migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of unimolecular expansion type alkyl phosphinate flame retardant of the present invention comprises the following steps:
[0030] 1) Hydrolysis acid production: 2-methyl-2,5-dioxo-1,2-oxophospholane is hydrolyzed to obtain methylpropionyl phosphinic acid; the solvent used in the hydrolysis reaction is water or acetone, Or a mixture of water and acetone; the temperature of the hydrolysis reaction is 40-100°C, and the reaction time is 0.5-3h;
[0031]2) Esterification reaction or esterification reaction and amidation reaction: carry out esterification reaction or esterification reaction and amidation reaction with methyl propionyl phosphinic acid and nitrogen-containing polyhydroxy compound to obtain nitrogen-containing ester-containing alkyl Phosphinic acid; wherein the nitrogen-containing polyhydroxy compound is one of the compounds having at least two hydroxyl groups, wherein the nitrogen-containing moiety can be amino or other nitrogen-containin...
Embodiment 1
[0036] Dissolve 1 mol of 2-methyl-2,5-dioxo-1,2-oxaphospholane in 5 mol of acetone at 50°C, slowly add 1 mol of water, stir and reflux for about 1 hour, filter and dry to obtain Methyl propionyl phosphinic acid; 3 mol of the above methyl propionyl phosphinic acid and 1 mol of 1,3,5-tris (2-hydroxyethyl) isocyanurate were added to a container containing 3 times the volume In a toluene flask, stir and reflux at 140°C for 4 hours, cool, pour off the upper layer solution to obtain colorless and transparent 1,3,5-tris(2-hydroxyethyl)isocyanurate methylpropionic acid Triester phosphinic acid; at normal temperature, add 1 times the volume of distilled water to the above 1mol nitrogen-containing ester-containing alkylphosphinic acid, stir until it dissolves into a homogeneous solution, then add NaOH aqueous solution dropwise to adjust the volume of the solution When the pH value reaches 7.0, 1,3,5-tris(2-hydroxyethyl)isocyanurate methyl propionate triester sodium phosphinate aqueous s...
Embodiment 2
[0049] Dissolve 1 mol of 2-methyl-2,5-dioxo-1,2-oxaphospholane in 5 mol of acetone at 40°C, slowly add 1 mol of water, stir and reflux for about 0.5 hours, filter and dry to obtain Methyl propionyl phosphinic acid; 3 mol of the above methyl propionyl phosphinic acid and 1 mol of 1,3,5-tris (2-hydroxyethyl) isocyanurate were added to a container containing 3 times the volume In a xylene flask, stir and reflux at 160°C for 2 hours, cool, pour off the upper solution to obtain colorless and transparent 1,3,5-tris(2-hydroxyethyl)isocyanurate methylpropane Acid triester phosphinic acid; at room temperature, add 1 times the volume of distilled water to the above 1 mol of nitrogen-containing ester-containing alkyl phosphinic acid, stir until it dissolves into a homogeneous solution, and then add NaOH aqueous solution dropwise to adjust the solution pH value to 7.0, to obtain 1,3,5-three (2-hydroxyethyl) isocyanurate methyl propionate triester sodium phosphinate aqueous solution; The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com