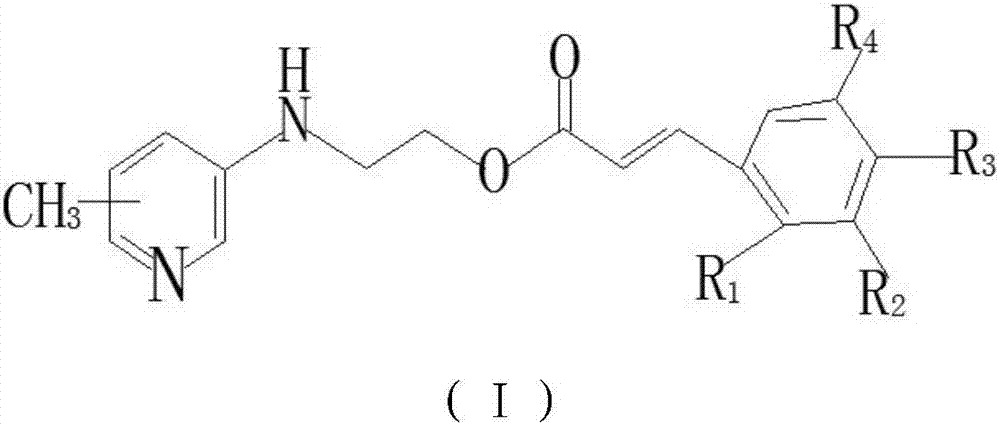
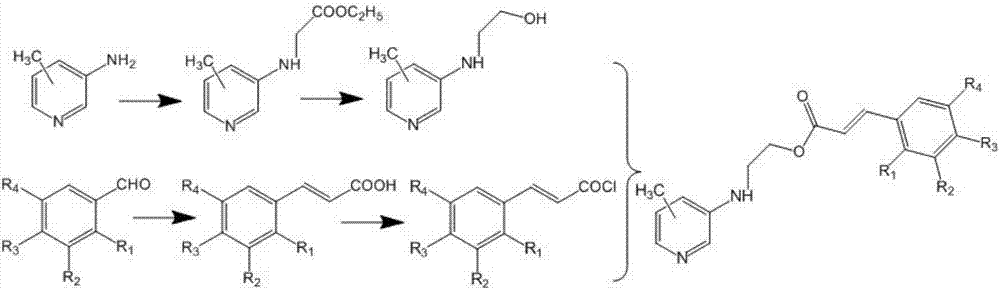
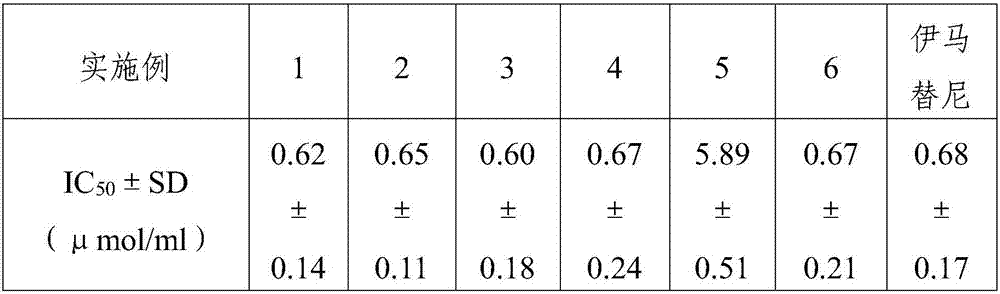
Aminomethylpyridine derivative, preparation method and application thereof
A technology of aminomethylpyridines and derivatives, which can be used in drug combination, organic chemistry, antitumor drugs, etc., can solve the problem of low anti-chronic cell leukemia activity, and achieve simple post-processing method, novel structure, and easy industrial operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (E)-3-(2-Chloro-4-trifluoromethylphenyl)-2-((4-methylpyridin-3-yl)amino)ethyl acrylate
[0026] (1) Dissolve 10.8g (0.10mol) of 3-amino-4-methylpyridine, 9.50ml (0.09mol) of ethyl chloroacetate and 14.45ml (0.10mol) of triethylamine in 40ml of tetrahydrofuran, reflux for 8h, and cool to room temperature, filtered, and the filtrate was rotary evaporated under reduced pressure, and the obtained concentrated solution was dissolved in ethyl acetate, extracted with dilute hydrochloric acid (pH=2) and sodium carbonate solution (pH=12) successively, and the organic phase was rotary evaporated under reduced pressure. , recovered ethyl acetate, dried to obtain 16.1 g of a light yellow oil, and the yield was 83.0%;
[0027] (2) Dissolve 9.70g (0.05mol) of the product of step (1) in 80ml of methanol, slowly add 4.24g (0.10mol) of lithium chloride and 3.78g (0.10mol) of sodium borohydride, react at 40°C for 5h, and add bicarbonate The sodium solution was filtered, the organic phas...
Embodiment 2
[0031] (E)-3-(3,4,5-trimethoxyphenyl)-2-((4-methylpyridin-3-yl)amino)ethyl acrylate
[0032] The operation steps are the same as in Example 1, and 3,4,5-trimethoxybenzaldehyde is used in step (3) instead of 2-chloro-4-trifluoromethylbenzaldehyde to obtain the target product as a yellow solid; the purity is 98.2%; 1 HNMR (300MHz, DMSO-d 6 )δ: 8.28(s, 1H); 8.05(s, 1H); 7.67(m, 1H); 7.48(s, 1H); 7.21(m, 1H); 6.91(d, 3H); ); 4.56(m, 2H); 3.56(m, 2H); 2.31(s, 3H); 3.83(s, 6H); 3.76(s, 3H); ESI-MS m / z(%): 372.2([ M+1] + ).
Embodiment 3
[0034] (E)-3-(2-Chloro-4-trifluoromethylphenyl)-2-((5-methylpyridin-3-yl)amino)ethyl acrylate
[0035] The operation steps are the same as in Example 1, and in step (1), 3-amino-5-picoline is used instead of 3-amino-4-picoline to obtain the target product as a light brown solid; the purity is 98.0%; 1 H NMR (300MHz, DMSO-d 6 )δ: 8.28(s, 1H); 8.05(s, 1H); 7.66(m, 2H); 7.30(m, 1H); 7.21(m, 1H); 6.90(d, 1H); ); 6.07(s, 1H); 4.56(m, 2H); 3.56(m, 2H); 2.23(s, 3H); ESI-MS m / z(%): 384.7([M+1] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com