Method for eliminating impurity influence of all-vanadium redox flow battery electrolyte
An all-vanadium redox flow battery and electrolyte technology, applied in the field of flow batteries, can solve the problems of inability to completely remove the influence of impurities, limited quantity and type of impurities, and increased cost of removing impurities, so as to achieve rich raw materials, simple methods, and extended periods of time. effect of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
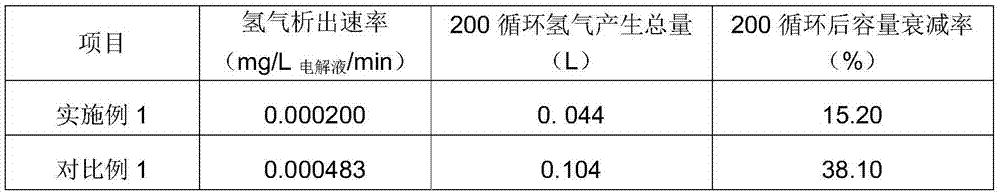
Embodiment 1
[0039] Electrolyte state: sulfate radical concentration 2.0mol / L; vanadium ion concentration 1.6mol / L; impurity elements and Ti ion content 600ppm; complexing agent composition: 2g sodium tripolyphosphate, solid; analytically pure; operating process: at ambient temperature Under the condition of 32°C; add 2g of sodium tripolyphosphate to the positive and negative electrolytes respectively, and stir evenly to fully complex the impurity ions with the complexing agent.
[0040] Battery power: 5W, test instrument: Arbin charge-discharge instrument; charge-discharge mode method: constant current charge-discharge mode; electrolyte solution amount: positive and negative electrodes 80mL each; charge-discharge cut-off voltage: 1.0-1.55V; current density: 80mA / cm 2 , after carrying out 200 charge-discharge cycles, (comparative example is the electrolytic solution that does not add complexing agent, other conditions are identical with embodiment)
[0041] The test results are as follow...
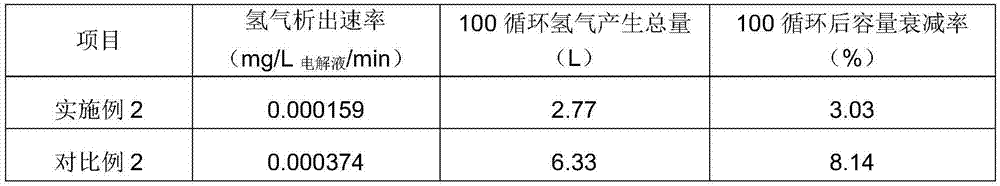
Embodiment 2
[0044]Electrolyte solution state: sulfuric acid concentration 1.5mol / L; hydrochloric acid concentration 1.0mol / L; vanadium ion concentration 1.6mol / L; impurity elements and content Cr ions 200ppm; complexing agent composition: 743g triethanolamine, solid; analytically pure; operating process : At an ambient temperature of 45°C; add 743g of triethanolamine into the positive and negative electrolytes respectively, and stir evenly to fully complex the impurity ions with the complexing agent.
[0045] Battery power: 1kW, test instrument: Arbin charge-discharge instrument; charge-discharge mode method: constant current charge-discharge mode; electrolyte solution amount: positive and negative electrodes 35L each; charge-discharge cut-off voltage: 10.0-15.5V; current density: 80mA / cm 2 , after carrying out 100 charge-discharge cycles, (comparative example is the electrolytic solution that does not add complexing agent, other conditions are identical with embodiment)
[0046] The te...
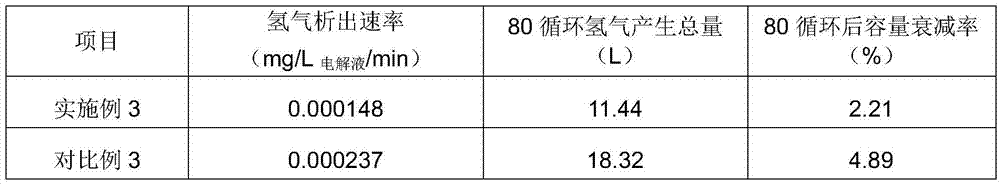
Embodiment 3
[0049] Electrolyte state: sulfuric acid concentration 2.0mol / L; vanadium ion concentration 1.6mol / L; impurity elements and Mn ion content 150ppm; complexing agent composition: 1875g sodium gluconate, solid; analytically pure; operating process: at an ambient temperature of 45 Under the condition of ℃; add 1875g of sodium gluconate to the positive and negative electrolytes respectively, and stir evenly to fully complex the impurity ions with the complexing agent.
[0050] Battery power: 20kW, test instrument: Arbin charge-discharge instrument; charge-discharge mode method: constant current charge-discharge mode; electrolyte solution amount: positive and negative electrodes 300L each; charge-discharge cut-off voltage: 52.0-80.6V; current density: 80mA / cm 2 , after carrying out 80 charge-discharge cycles, (comparative example is the electrolytic solution that does not add complexing agent, other conditions are identical with embodiment)
[0051] The test results are as follows:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com