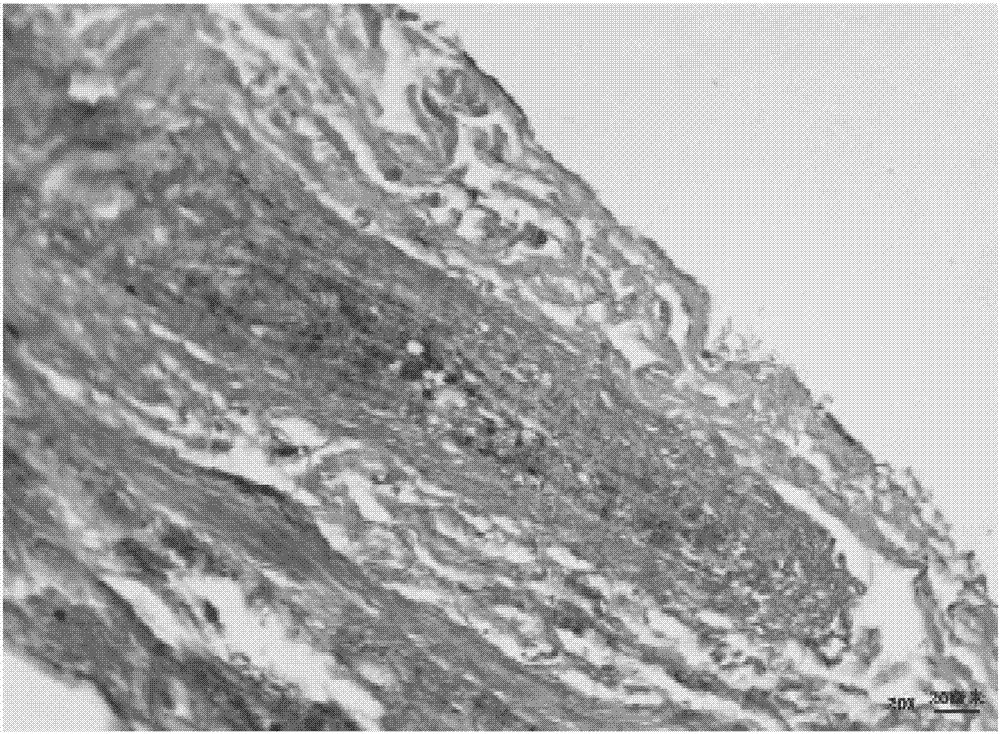
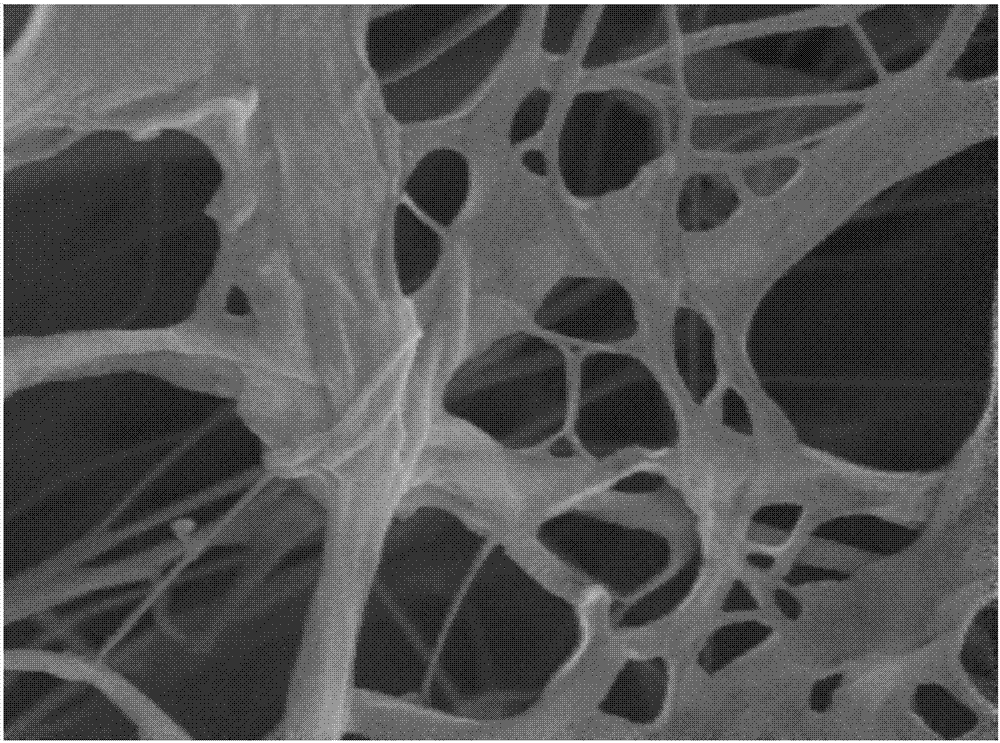
Biological tissue matrix material, and preparation method and purpose thereof
A technology of biological tissue matrix and tissue materials, applied in tissue regeneration, medical science, prosthesis, etc., can solve problems that affect the product's anti-infection ability, tissue regeneration ability, destruction of three-dimensional structure, product instability, etc., to reduce the risk of occurrence , Improve the bonding strength, improve the effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] This embodiment relates to a method for preparing an animal-derived implantable biological patch, including the following steps:
[0048] (1) Primary treatment of raw materials:
[0049] Take porcine or bovine small intestinal submucosa tissue material (porcine small intestinal submucosa tissue material is also called porcine small intestinal submucosa SIS) and divide it into a specified size of 8 cm in width and 15 cm in length, remove useless tissues (such as lymphoid tissue), and rinse with tap water for 1- 3 times, and then rinse with purified water until there is no stain on the surface, place the rinsed porcine or bovine small intestinal submucosa tissue material in a water filtration device such as a filter for more than 5 minutes, and drain the water. When taking the small intestine, measure the filtered small intestine with a volume measuring device such as a measuring cylinder.
[0050] (2) Virus inactivation:
[0051] Use peracetic acid-ethanol solution to ...
Embodiment 2
[0059] This embodiment relates to a method for preparing an animal-derived implantable biological patch, including the following steps:
[0060] (1) Primary treatment of raw materials:
[0061] Take pig or bovine small intestinal submucosa tissue material and divide it into specified size 5cm wide and 15cm long, remove useless tissue (such as lymphoid tissue), rinse with purified water until there is no stain on the surface, and wash the pig or bovine small intestinal submucosa material Put it in a water filter device such as a strainer for more than 5 minutes, and drain the water. When taking the small intestine, measure the filtered small intestine with a volume measuring device such as a measuring cylinder.
[0062] (2) Virus inactivation:
[0063] Use peracetic acid-ethanol solution to soak porcine or bovine small intestinal submucosa tissue material for virus inactivation, and this process can be carried out in a stainless steel barrel. The concentration of peracetic a...
Embodiment 3
[0071] The manufacturing method involved in the present invention may also include the following steps:
[0072] (6) Fixed molding:
[0073] Carry out on the mould, said mould includes needle base plate and pressure frame, need to select different molds according to different specifications and sizes, the pig or bovine small intestinal submucosa matrix material prepared in step (5) is spread on the needle base plate , placing the pressure frame on the pig or bovine small intestine submucosa matrix material, the size of the pressure frame can be the final cut size or wider, and relatively fixing the needle base plate and the pressure frame.
[0074] (7) Vacuum freeze drying:
[0075] It is carried out in a vacuum freeze-drying machine. The freeze-drying process of the product needs to be reconfirmed according to different equipment. The mold is laid flat in the vacuum freeze-drying machine, the door of the freeze-drying chamber is closed, the circulation pump is turned on for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Burst strength | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com