Coordination balance cationic salt, and magnet formed by metal dithiolene complex and preparation methods thereof
A technology of cationic salts and complexes is applied in the fields of coordination balance cationic salts and magnets formed by metal disulfide complexes and their preparation, and achieves the effects of being beneficial to spin coupling and electron transport.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
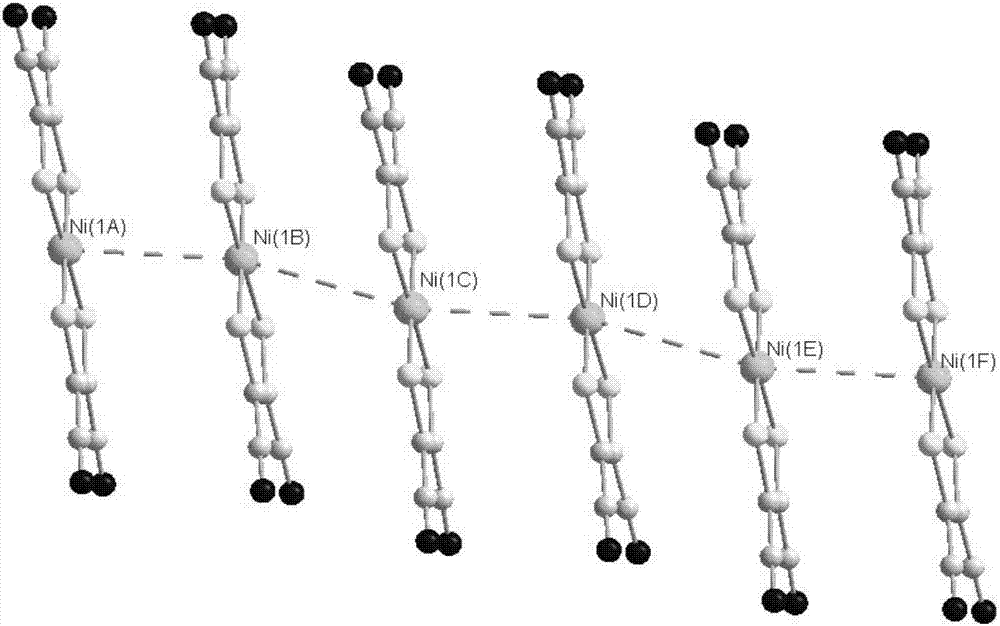
[0046] The present invention provides a magnet formed from a metal disulfide complex represented by formula (II),
[0047]
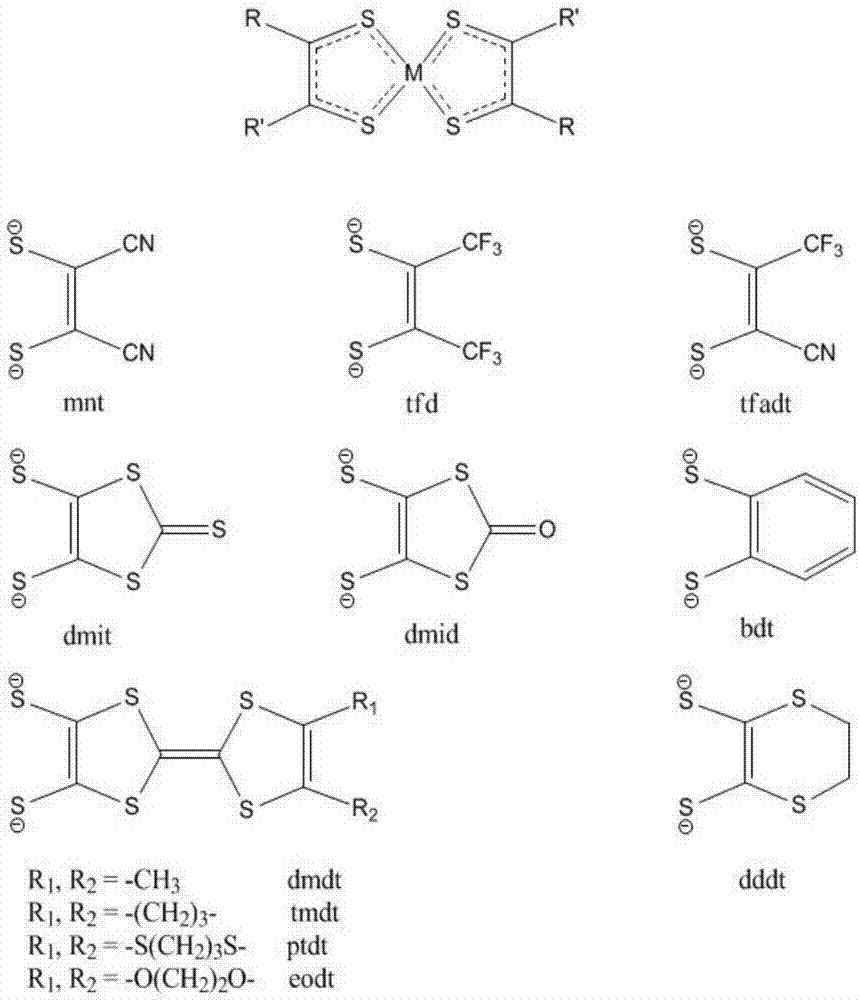
[0048] Among them, the mnt is
[0049] The preparation route of the compound shown by formula (II) is:
[0050]
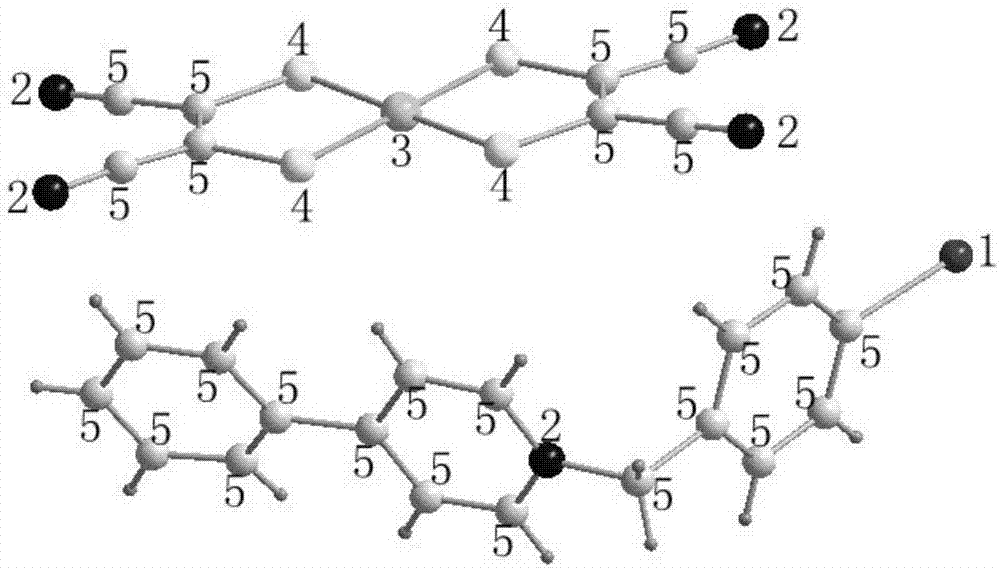
[0051] S1: Dissolve 1.55g (0.01mol) of 4-phenylpyridine and 2.97g (0.01mol) of 4-iodobenzyl bromide in 25mL of acetone, heat to reflux in an oil bath for 6 hours, let stand for 0.5h, filter, and wash with acetone , to obtain 4-iodobenzylphenylpyridinium bromate, which is the coordination balance cation salt shown by formula (I);
[0052] S2: Dissolve 30.0 g (0.61 mol) of sodium cyanide in the first batch of N, N-dimethylformamide 100 mL to obtain a solid-liquid mixture, then add the second batch of N, N-dimethylformamide 80 mL, Fully grind the solid-liquid mixture in batches, then add 34.4g (0.45mol) of carbon disulfide, the solution turns brownish black, and react with magnetic stirring at room temperature; hour; then add 100mL of...
Embodiment 2
[0057] The present invention provides a magnet formed from a metal disulfide complex represented by formula (II),
[0058]
[0059] Among them, the mnt is
[0060] The preparation route of the compound shown by formula (II) is:
[0061]
[0062] S1: Dissolve 3.10g (0.02mol) of 4-phenylpyridine and 6.88g (0.02mol) of p-iodobenzyl iodide in 50mL of acetone, heat to reflux in an oil bath for 6 hours, let stand for 0.5h, filter, and wash with acetone , to obtain 4-iodobenzylphenylpyridinium iodate, which is the coordination balance cation salt shown by formula (I);
[0063] S2: Dissolve 30.0 g (0.61 mol) of sodium cyanide in the first batch of N, N-dimethylformamide 100 mL to obtain a solid-liquid mixture, then add the second batch of N, N-dimethylformamide 80 mL, Fully grind the solid-liquid mixture in batches, then add 46.4g (0.61mol) of carbon disulfide, the solution turns brownish black, and react with magnetic stirring at room temperature; Then add 100mL of anhydro...
Embodiment 3
[0068] The present invention provides a magnet formed from a metal disulfide complex represented by formula (II),
[0069]
[0070] Among them, the mnt is
[0071] The preparation route of the compound shown by formula (II) is:
[0072]
[0073] S1: Dissolve 0.78g (0.005mol) of 4-phenylpyridine and 1.26g (0.005mol) of 1-(chloromethyl)-4-iodobenzene in 12.5mL of acetone, heat and reflux in an oil bath for 6 hours, and let stand 0.5h, filter, adopt acetone to wash, obtain 4-iodobenzylphenylpyridinium chlorate, promptly by the coordination balance cation salt shown in formula (I);
[0074] S2: Dissolve 30.0 g (0.61 mol) of sodium cyanide in the first batch of N, N-dimethylformamide 100 mL to obtain a solid-liquid mixture, then add the second batch of N, N-dimethylformamide 80 mL, Fully grind the solid-liquid mixture in batches, then add 40.4g (0.53mol) of carbon disulfide, the solution turns brownish black, and react with magnetic stirring at room temperature; Then add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com