Preparation method of varicella-zoster virus glycoprotein E extracellular domain protein
A herpes zoster virus and extracellular region technology, which is applied in the field of preparation of varicella-zoster virus glycoprotein E extracellular region protein, can solve the problem that protein immunogenicity is not closely related to glycosylation, and protein glycosylation is not closely related. Inconsistency, protein heterogeneity and other problems, to achieve the effect of good immunogenicity, good immunogenicity and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
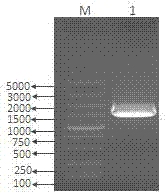
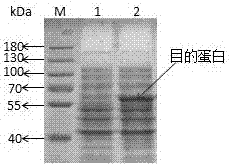
Image
Examples
Embodiment 1
[0042] Preparation of varicella-zoster virus glycoprotein E protein antigen
[0043] 1. Target gene amplification
[0044] (1) Primer design
[0045] According to the varicella-zoster virus glycoprotein E gene sequence published on Genbank, select the nucleic acid sequence corresponding to the amino acid sequence 25-537 to design primers with Nhe I and EcoR I restriction sites (marked in italics) at both ends:
[0046] The forward primer is (5'-3'): TCCTA GCTAGC AT GACCAATCCGGTTCGCGCCAGCGTGC;
[0047] The reverse primer is (5'-3'): TTCCG GAATTC TTAATGATGATGATGATGATGACGCAGC
[0048] AGCGGGCTTGTACCCGGAT;
[0049] (2) PCR amplification
[0050] Using the synthesized plasmid as a template, use the above primers for nucleic acid amplification. The specific reaction system is PrimeSTAR HSDNA polymerase buffer (5×) 10ul, dNTPs 4ul, Primer-F 1ul, Primer-R 1ul, template 1ul, PrimeSTAR HS DNA polymerase 0.5 ul, add deionized water to a total volume of 50ul; put the reaction tube...
Embodiment 2
[0073] Preparation of Protein Antigen of Varicella-Zoster Virus Glycoprotein E Extracellular Domain
[0074] (1) Primer design
[0075] According to the varicella-zoster virus glycoprotein E gene sequence published on Genbank, select the nucleic acid sequence corresponding to the amino acid sequence 25-539 to design primers with Nhe I and EcoR I restriction sites (marked in italics) at both ends:
[0076] The forward primer is (5'-3'): TCCTA GCTAGC ATGACCAATCCGGTTCGCGCCAGCGTGC; reverse primer is (5'-3'): TTCCG GAATTC TTAATGATGATGATGATGATGCGCATAACGCAGCAGCGGGCTTGTAC;
[0077] (2) Ligation of PCR product and cloning vector
[0078] Amplify the target fragment according to the designed primers, after recovery, carry out enzyme digestion with the carrier, quantify the target gene and carrier enzyme digestion products, take 12ul and 2ul respectively, add 10× T4 ligase buffer 2ul, T4 ligase 1ul , add deionized water to 20ul, and connect overnight at 16°C to obtain the recombinan...
Embodiment 3
[0085] Preparation of Protein Antigen of Varicella-Zoster Virus Glycoprotein E Extracellular Domain
[0086] 1. Primer design
[0087] According to the varicella-zoster virus glycoprotein E gene sequence published on Genbank, select the nucleic acid sequence corresponding to the amino acid sequence 25-546, and design primers with Nhe I and EcoR I restriction sites (marked in italics) at both ends,
[0088] The forward primer is (5'-3'): TCCTA GCTAGC ATGACCAATCCGGTTCGCGCCAGCGTGC;
[0089] The reverse primer is (5'-3'): TTCCG GAATTC TTAATGATGATGATGATGATGGCCCAGG
[0090] CCCGCGGTCCACGCCGCA;
[0091] 2. Ligation of PCR product and cloning vector
[0092] Amplify the target fragment according to the designed primers, after recovery, carry out enzyme digestion with the carrier, quantify the recovered products of the target gene and carrier enzyme digestion, take 12ul and 2ul respectively, add 10× T4 ligase buffer 2ul, T4 ligase 1ul, Add deionized water to 20ul, and connect ov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com