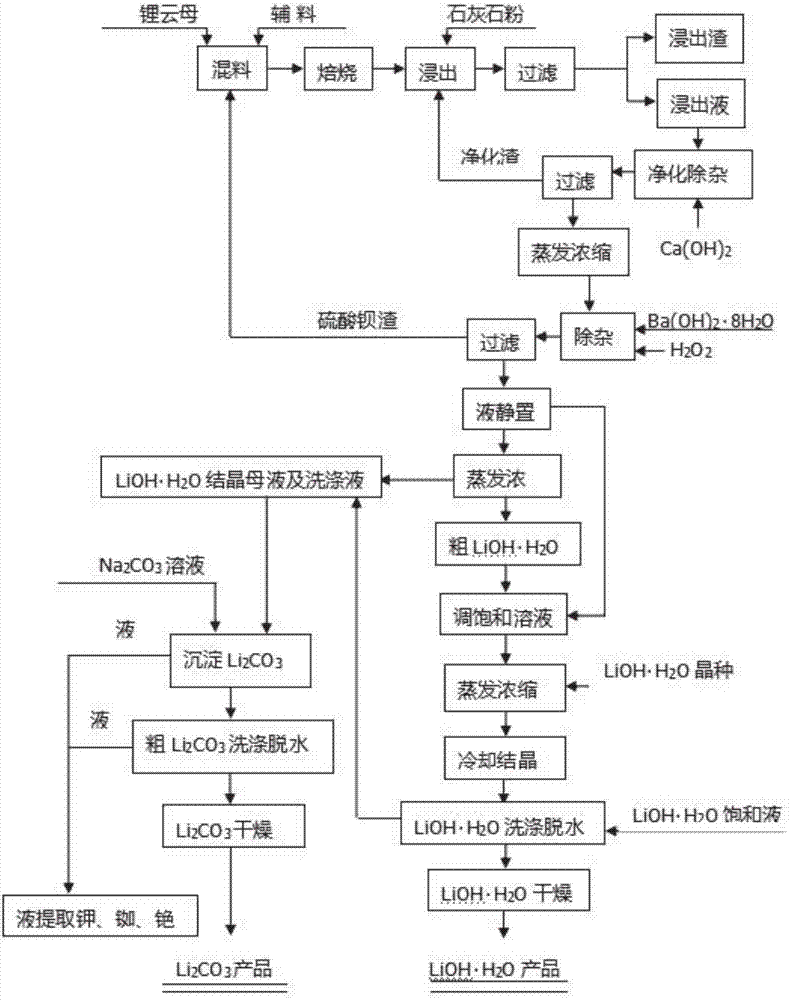
Method for extracting lithium from lepidolite concentrate
A lepidolite and lithium extraction technology, which is applied in the direction of lithium oxide;/hydroxide, lithium carbonate;/acid carbonate, etc., can solve the problems of easy formation of saturated double salt, melting of materials, and low lithium grade and other problems, to achieve the effect of solving the dust return of roasted materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Step (1): 2 kg of lepidolite concentrate (see Table 1 for composition): industrial grade CaSO 4 2H 2 O: Barite ore powder (containing BaSO 4 ≥90%): anthracite coal powder (calorific value Q=5000 kcal / kg) by weight ratio 1:0.3:0.15:0.1, add 3% water of the total material and mix evenly, and press it into a green ball of φ25mm in a small ball press machine , put it into a stainless steel pan, and roast it in a muffle furnace at 920°C for 2h (stirring once every 30 minutes). It was observed that the roasted pellets tended to soften and melt but did not melt, and the roasted pellets were obtained.
[0125] Step (2): The obtained roasted pellets are ground in a vibration mill to -180 mesh, accounting for 80% of the powder, and water is adjusted to a slurry in a reaction tank at a solid-to-liquid ratio of 1:2, and then mixed according to ore:acid (weight ratio) = 1:0.5 ratio, add concentrated sulfuric acid and react without heating for 2 hours, then add limestone powder (Ca...
Embodiment 2
[0130] Compared with Example 1, the difference is that in step (1), lepidolite concentrate (see Table 1 for composition) 2kg: industrial grade CaSO 4 2H 2 O: the BaSO produced by the experiment of Example 1 4 Return slag (including BaSO 4 ≥90%), anthracite coal powder (calorific value Q=5000 kcal / kg), add 3% water of the total material in a weight ratio of 1:0.2:0.2:0.04, mix evenly, and press into a green ball of φ25mm in a small ball press , put it into a stainless steel pan, and roast it in a muffle furnace at 950° C. for 2 hours (stirring once every 30 minutes). It was observed that the roasted material did not soften and melt, and the roasted material was obtained. Subsequent process is the same as in Example 1, and the results are shown in Table 2.
Embodiment 3
[0132] Compared with Example 1, the difference is that in step (1), the calcination temperature is 850°C. Subsequent process is the same as in Example 1, and the results are shown in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com