Fusion protein 210 and applications thereof in virus replication optimization
A fusion protein and virus replication technology, which is applied in the field of virus isolation and identification and whole virus vaccine preparation, can solve the problems of obvious cytotoxicity, few reports in the field of virology, cell apoptosis, etc., and achieve high expression efficiency and time-consuming detection Shortened, easy-to-purify effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Prokaryotic expression and purification of fusion protein 210 gene
[0036] Using E. coli engineered bacteria to express fusion protein 210, the specific method includes the following steps:
[0037] 1. Gene cloning: Using glioma tumor suppressor candidate gene 2 (GenBank: KJ898763.1) as a template, design primers for subcloning of the target gene; among them, the upstream primer: 5'-CGAGGTCTGTCCCACGCCCG-3', the downstream primer: 5 '-CAGCTCCGAGCTCAGCTGCA-3';
[0038] 2. Vector construction: cut the pET-28a vector with restriction enzymes NdeI / EcoRI, cut the target gene obtained in step 1) and ligate it with the vector to obtain the recombinant vector pET-210 containing the target gene;
[0039] 3. Cell transformation and expression: Transform BL21(DE3) with recombinant vector pET-210, and culture to OD at 37°C on a shaker 590 0.8-1.0, add inducer IPTG to a final concentration of 1mM, and continue to incubate at 37°C for 4h;
[0040] 4. Protein purification: the induce...
Embodiment 2
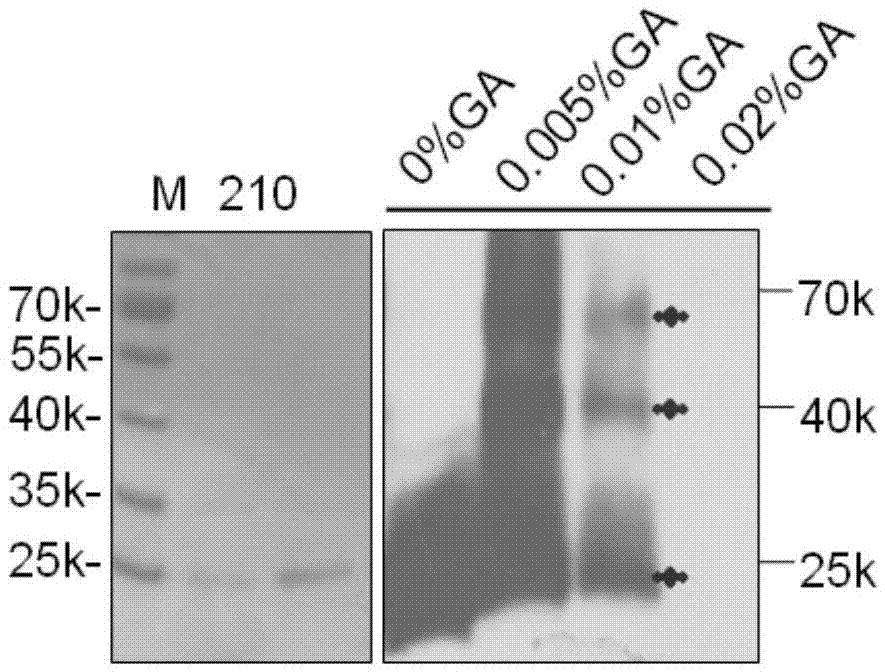
[0047] Example 2 Study on the effect of fusion protein 210 on virus virulence by immunoblotting, cytopathic and qRT-PCR
[0048] Western-blot: Wash the virus-infected cells with pre-cooled pH 7.5 PBS for 3 times, scrape the cells, lyse the cells with ultrasound and extract the total protein for 3-5 seconds, and compare them to 6×SDS-PAGE The sample buffer was mixed, boiled for 10 minutes, and SDS-PAGE electrophoresis was performed. After electrophoresis, the protein on the gel was transferred to the PVDF membrane by an electro-membrane transfer machine. Block with PBST buffer containing 5% skimmed milk powder for 1 hour at room temperature, and add primary antibody to incubate for 2 hours with shaking at 4°C. Wash the membrane 4 times with PBST, 5 min each time, and incubate the corresponding HRP-labeled secondary antibody with 1:8000 dilution at room temperature for 1 h, wash the membrane 4 times, 5 min each time, and finally develop the color with SuperSignal West Pico Chemilum...
Embodiment 3
[0065] Example 3 Cytotoxicity test of fusion protein 210
[0066] Lactate dehydrogenase (LDH) experiment is used to study whether the polypeptide or protein is toxic to cells, and it is completed with a toxicity detection kit purchased from Roche. Add the following final concentrations of fusion protein 210 to CEF cells cultured to a monolayer: 5μM, 25μM, 50μM, 100μM, 250μM, 500μM, 1.0mM, and gently mix them evenly. After 24 hours, determine the toxicity index according to the kit instructions . The experiment was repeated three times. The results showed that the fusion protein 210 had no toxic effect on cells at a concentration of 50 μM and below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com