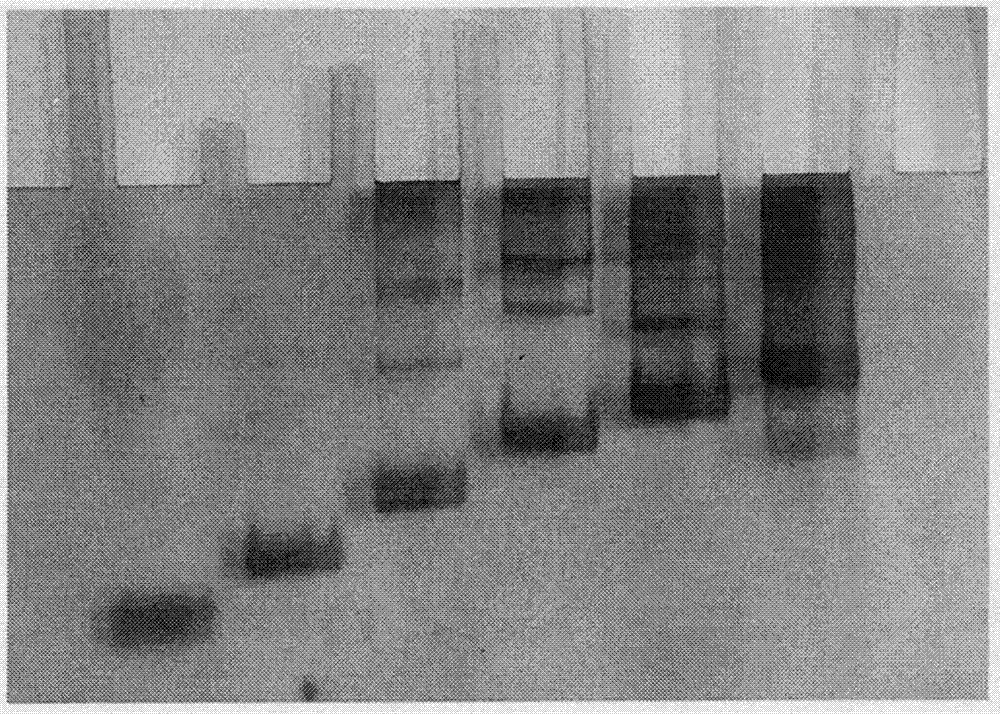
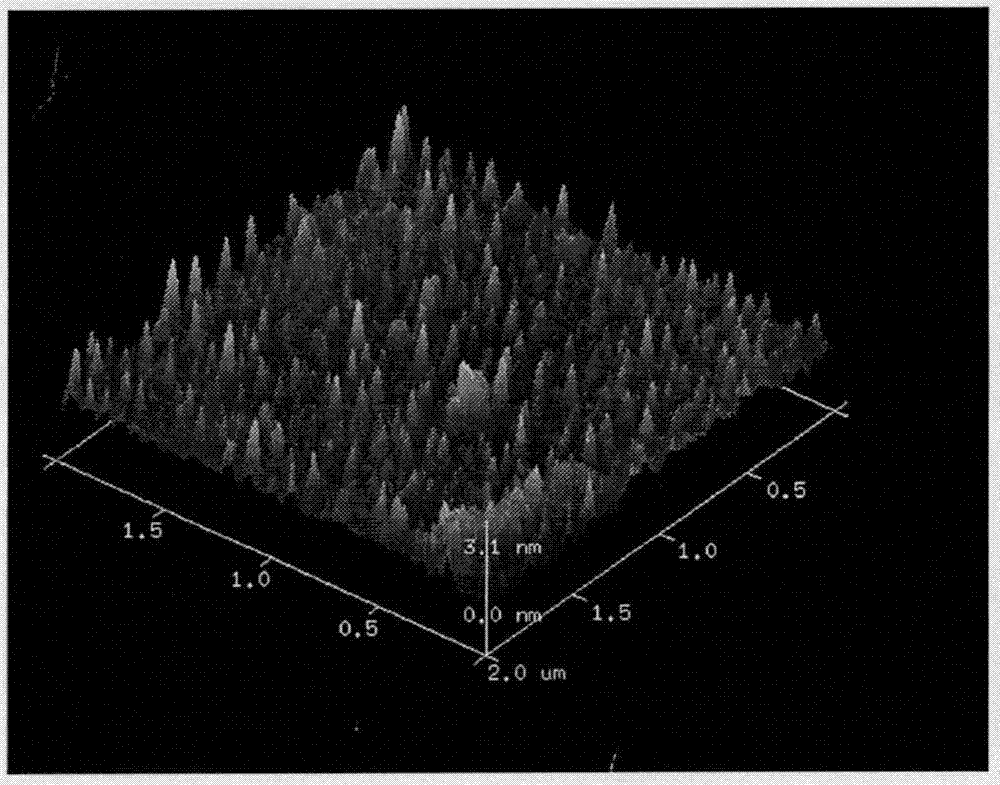
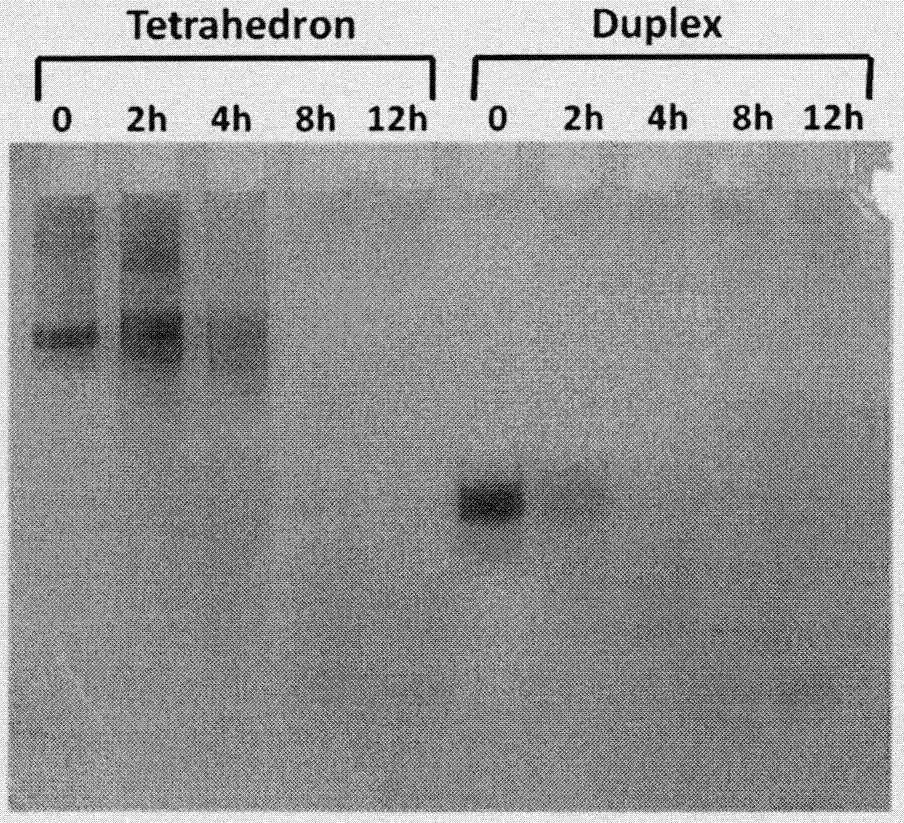
Adriamycin-loaded aptamer-modified DNA nanocage and preparation method thereof
A nanocage and aptamer technology, applied in the field of medicine, can solve the problems of limiting tumor treatment effect, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0025] step 1:
[0026] Send the following 7 DNA oligonucleotide strands to the company for synthesis:
[0027] A: 5′-CGT ATC ACC AGG CAG TTG AGA CGA ACA TTC CTA AGT CTG AA A TTT ATCACC C GC CAT AGT AG-3′
[0028] B: 5′-CGA TTA CAG CTT GCT ACA CGA TTC AGA CTT AGG AAT GTT CGA CAT GCGAGG GTC CAA TAC CG-3′
[0029] C: 5′-CGT GTA GCA AGC TGT AAT CGA CGG GAA GAG CAT GCC CAT CCA CTA CTATGG CGG GTG ATA AA-3′
[0030] D: 5′-CTC AAC TGC CTG GTG ATA CGA GGA TGG GCA TGC TCT TCC CGACGG TATTGG ACC CTC GCA TG-3′
[0031] A′: 5′-CGT ATC ACC AGG CAG TTG AGA CGA ACA TTC CTA AGT CTG AA A TTTATC ACC C GC CAT AGT AGTTTTTTTT GCAGTTGATCCTTTGGATACCCTGG-3′
[0032] B′: 5′-CGA TTA CAGCTT GCT ACA CGA TTC AGA CTT AGG AAT GTT CGA CAT GCGAGG GTC CAA TAC CGTTTTTTTT GCAGTTGATCCTTTGGATACCCTGG-3′
[0033]C′: 5′-CGT GTA GCA AGC TGT AAT CGA CGG GAA GAG CAT GCC CAT CCA CTA CTATGG CGG GTG ATA AATTTTTTT GCAGTTGATCCTTTGGATACCCTGG-3′
[0034] Use TM buffer (10mM Tris-HCl, 5mMMgCl 2 , pH 8.0) was dissolved for ...
specific Embodiment 2
[0038] Human liver cancer Hep G2 cells with low MUC1 expression were used as a negative control to conduct an in vitro cell uptake test to investigate the cell-targeted uptake capabilities of Td, apt-Td, 2apt-Td, and 3apt-Td obtained in Example 1.
[0039] Human breast cancer MCF-7 cells and human liver cancer HepG2 cells were mixed at a density of 1×10 5 Cells / well were seeded in 24-well plates and cultured for 24 hours. Then, the cells were treated with serum-free medium containing samples (Td, apt-Td, 2apt-Td and 3apt-Td obtained in Example 1, the same final concentration was 1000 nM) for 5 h (37° C.). After the cells were incubated for 4 hours, they were washed 3 times with fresh PBS, and then the cells were digested with trypsin. After the digestion was completed, PBS was added to make a cell suspension. After passing through a microporous membrane, the uptake ability of the cells was observed by flow cytometry. The results are attached Figure 4 shown. The results sho...
specific Embodiment 3
[0041] Dox@Td, Dox@apt-Td, Dox@2apt-Td, Dox@3apt-Td obtained in Example 3 of the present invention were subjected to in vitro cell uptake experiments.
[0042] Human breast cancer MCF-7 cells were cultured at a density of 1×10 5 Cells / well were seeded in 24-well plates and cultured for 24 hours. Then, cells were treated with samples containing (Dox@Td obtained in Example 3, Dox@apt-Td, Dox@2apt-Td, Dox@3apt-Td, and free Dox, the same final concentration of doxorubicin was 11.6 μg / mL) The serum-free medium was treated for 1, 2 and 3 hours (37°C) respectively. After the cells were incubated for a fixed time, they were washed 3 times with fresh PBS, and the uptake ability of the cells was observed by an inverted fluorescence microscope. The results are attached Figure 5 shown. The results showed that the cell uptake of free doxorubicin was the strongest at 1 h, and the cell entry ability of Dox@Td, Dox@apt-Td, Dox@2apt-Td, Dox@3apt-Td increased with the increase of the numbe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com