Kit for detecting swine mycoplasmal pneumonia based on direct PCR (Polymerase Chain Reaction) and application of kit
A technology of mycoplasma swine pneumonia and a kit, which is applied in the biological field, can solve the problems of inaccurate results, poor sensitivity, time-consuming and labor-intensive problems, and achieve the effects of strong specificity, improved detection, and improved work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The direct PCR detection kit of the swine mycoplasma pneumonia pathogen of the present embodiment, composition comprises:
[0019] (1) Lysis solution: 2-4M isothiocyanate, 7-10M ethylenediaminetetraacetic acid, 15-20M lauryl sarcosine, 3-6% (volume ratio) polyvinylpyrrolidone.
[0020] (2) PCR amplification reagent: 5×PCR Buffer, 2.0M dNTP, 10pM forward primer, 10pM reverse primer, high-fidelity Taq enzyme (5U / μl), 20mM MgCl 2 ,pure water.
[0021] The specific composition of each ingredient:
[0022] The reaction system is 25 μl, the lysed sample is 5 μl, MPSF (5′-GAAGCTATCAAAAAAGGGGAAACTA-3′) (10 pmol / μl) 2 μl, MPSR (3’-TGTATCGGTTTCAGAAGAAG-5′) (10 pmol / μl) 2 μl, 5×PCR Buffer 5 μl , 2.0mM / L dNTPs 5μl, high-fidelity Taq enzyme 0.5μl (5U / μl), 20mM MgCl 2 3.5μl, 2μl purified water, mix thoroughly and centrifuge briefly.
[0023] One pair of specific primers is as follows: MPSF: GAAGCTATCAAAAAAGGGGAAACTA, MPSR: TGTATCGGTTTCAGAAGAAG.
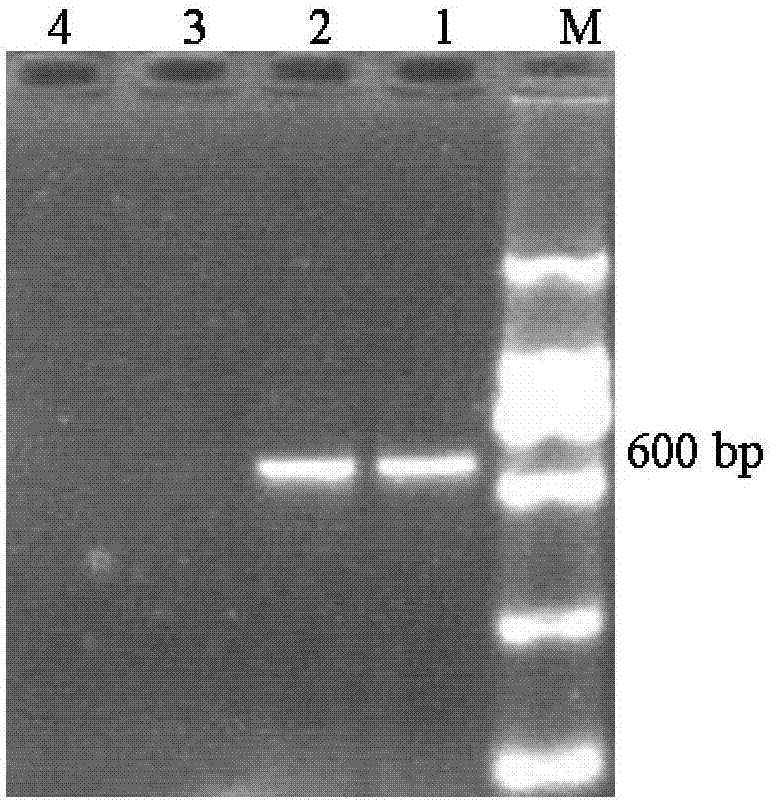
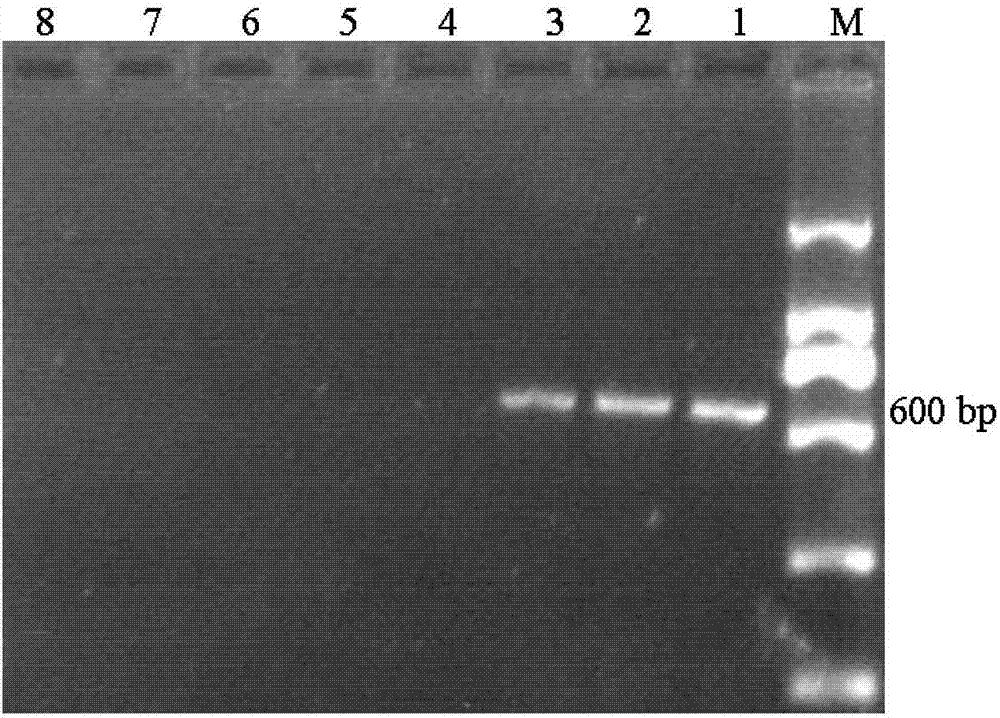
[0024] The kit is used to direc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com