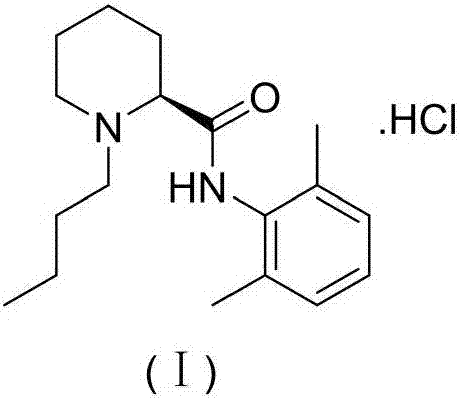
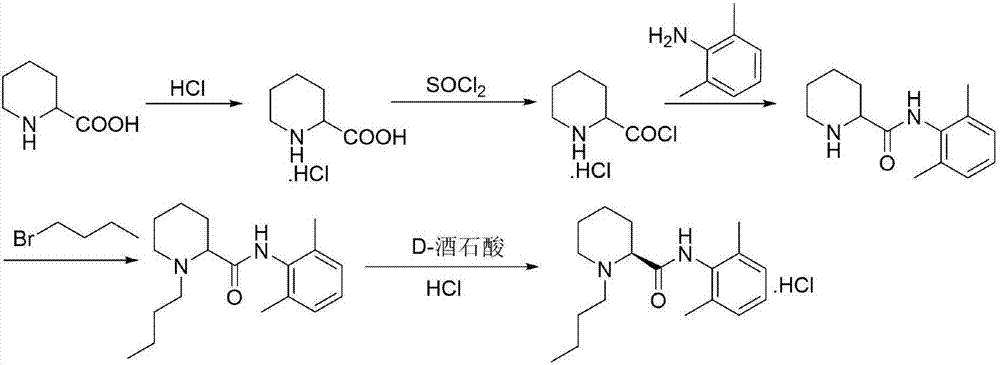
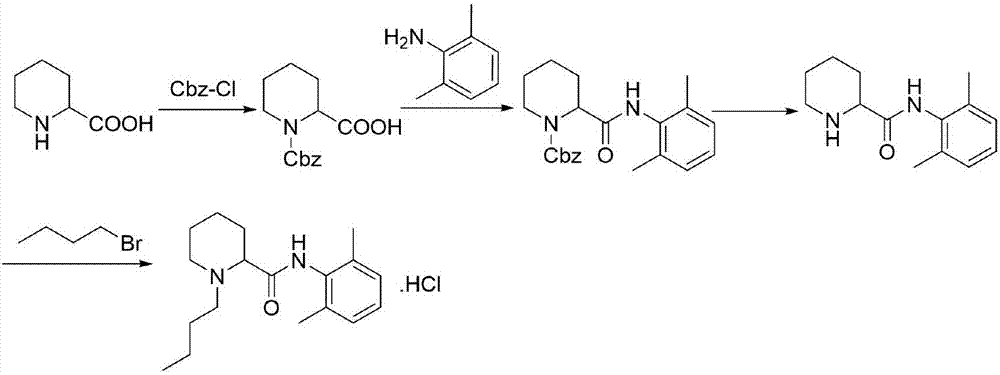
Preparation method of levobupivacaine hydrochloride
A technology of levobupivacaine hydrochloride and alcoholic hydrochloride solution is applied in the field of preparation of levobupivacaine hydrochloride, can solve the problems of harsh reaction conditions, prolonged preparation process route, increased production cycle and the like, and achieves mild reaction conditions and synthetic The route is short and avoids the effect of strong corrosiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Preparation of S configuration 1-butylpiperidine-2-carboxylic acid
[0043] Add 20g (0.155mol) of S-configuration 2-piperidinecarboxylic acid and 11.17g (0.155mol) of n-butyraldehyde into 200ml of methanol solvent, stir for about 1 hour, weigh 1g of palladium carbon with a mass fraction of 10%, and add it to the reaction solution , into hydrogen, after 3 times of gas exchange, the pressure rose to 20 atm, kept the pressure at room temperature for 1 to 2 hours, and terminated the reaction after testing that the reaction was complete. The reaction solution was filtered and the filtrate was collected. After the filtrate was spin-dried, 200 ml of petroleum ether was added to make a slurry, and the filter cake was filtered and collected. After drying, 27.5 g of S-configuration 1-butylpiperidine-2-carboxylic acid was obtained. Yield 95.7%. MS:184[M-H]
Embodiment 2
[0044] 1H-NMR(400MHz,DMSO-d6),ppm:3.26-3.23(m,1H),3.12-3.08(m,1H),3.0-2.9(m,2H),1.91-1.87(m,1H),1.63 -1.50(m, 6H), 1.38-1.35(m, 1H), 1.29-1.23(m, 2H), 0.89-0.86(t, J=7.2Hz, 3H) Example 2: Preparation of S configuration 1-butyl Piperidine-2-carboxylic acid
[0045] Add 20g (0.155mol) of S-configuration 2-piperidinecarboxylic acid and 33.53g (0.465mol) of n-butyraldehyde to 200ml of isopropanol solvent, stir for about 1 hour, weigh 10g of Raney nickel and add it to the reaction solution, Hydrogen gas was introduced, and the pressure rose to 5 atm after three gas exchanges, and the reaction was maintained at room temperature for 5 to 6 hours, and the reaction was terminated after testing that the reaction was complete. The reaction solution was filtered and the filtrate was collected. After the filtrate was spin-dried, 200ml of petroleum ether was added to make a slurry, and the filter cake was filtered and collected. After drying, 27.3g of S-configuration 1-butylpiperidine-2-ca...
Embodiment 3
[0046] Example 3: Preparation of racemic 1-butylpiperidine-2-carboxylic acid
[0047]Add 20g (0.155mol) of 2-piperidinecarboxylic acid and 16.8g (0.233mol) of n-butyraldehyde to 200ml of ethanol solvent, stir for about 1 hour, weigh 2g of palladium carbon with a mass fraction of 5%, and add it to the reaction solution. Introduce hydrogen and exchange gas three times, then continue to infuse hydrogen, react at room temperature and normal pressure for 8 to 10 hours, and terminate the reaction after testing that the reaction is complete. The reaction solution was filtered and the filtrate was collected. After the filtrate was spin-dried, 200ml of petroleum ether was added to make a slurry, and the filter cake was filtered and collected. After drying, 27.8g of 1-butylpiperidine-2-carboxylic acid was obtained. Yield 96.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com