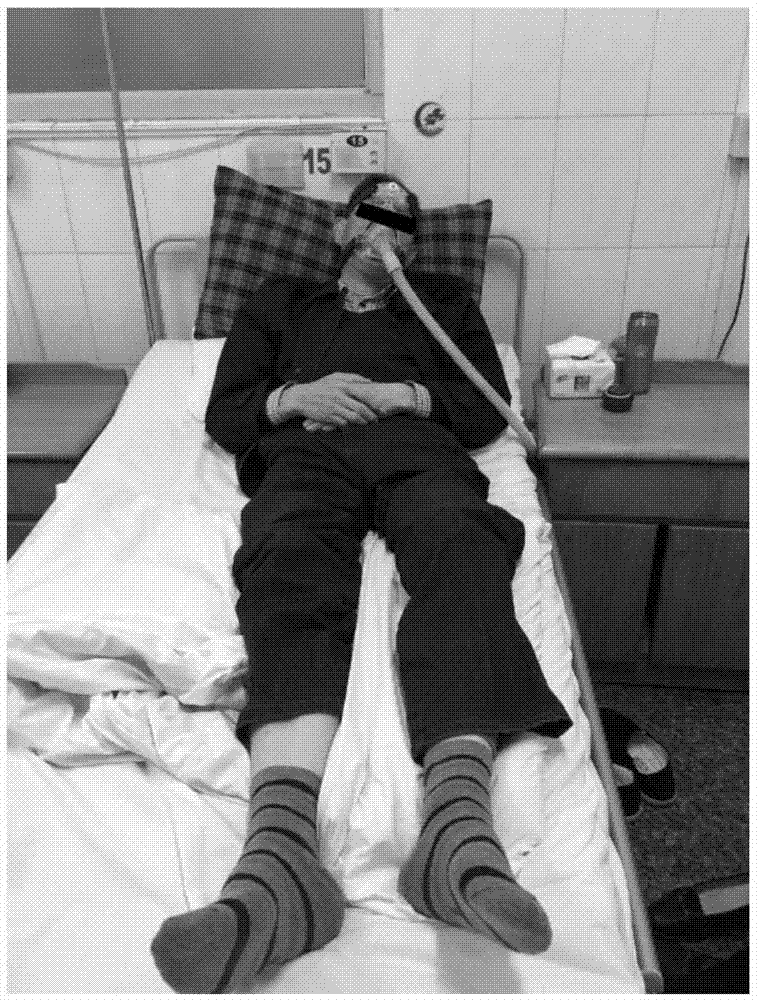
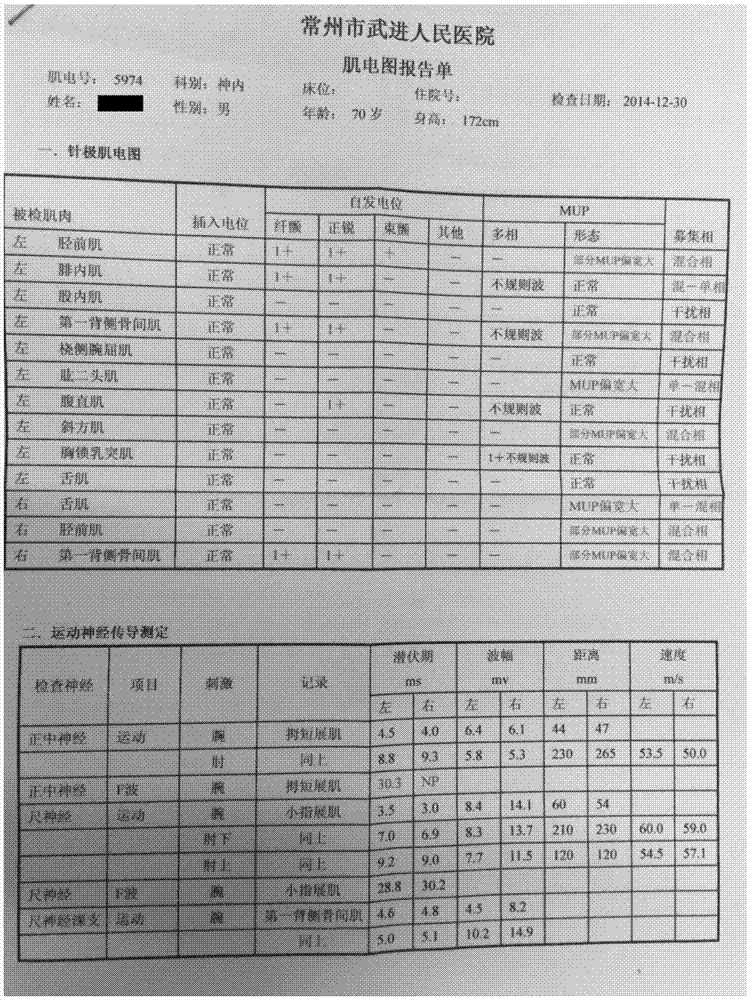
Composition for treatment of motor neuron disease and use thereof
A motor neuron disease and composition technology, which is applied in nervous system diseases, neuromuscular system diseases, drug combinations, etc., can solve the problem of safe, effective and low-cost drugs or health care products for motor neuron disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A pharmaceutical composition comprising L-ornithine hydrochloride equivalent to 2.5 g of L-ornithine, 1.5 g of aspartic acid and vitamin B 6 10g can be added to 1000mL of 5% glucose sodium chloride injection.
Embodiment 2
[0055] A pharmaceutical composition, its component is compound amino acid injection 1000mL (containing: L-ornithine hydrochloride equivalent to L-ornithine 3.5g, aspartic acid 2.50g, arginine 8.80g, Isoleucine 8.80g, Leucine 13.60g, Lysine Acetate Equivalent to Lysine 7.51g, Methionine 1.20g, Phenylalanine 1.60g, Threonine 4.60g, Tryptophan 1.50g , valine 10.60g, histidine 4.70g, glycine 6.30g, alanine 8.30g, proline 7.10g, asparagine 0.55g, N-acetyl-L- equivalent to cysteine 0.60g cysteine, glutamic acid 5.70g, serine 3.70g, N-acetyl-L-tyrosine equivalent to tyrosine 0.70g), and vitamin B 6 16g and vitamin C 3g, vitamins were added to 500mL of 0.9% sodium chloride injection.
Embodiment 3
[0057] A pharmaceutical composition, its component is compound amino acid injection 1000mL (containing: the L-ornithine hydrochloride equivalent to L-ornithine 4.5g, aspartic acid 2.80g, arginine 8.30g, Isoleucine 6.50g, Leucine 12.00g, Lysine Acetate Equivalent to Lysine 7.51g, Methionine 1.60g, Phenylalanine 1.40g, Tryptophan 1.80g, Valine 10.60g , histidine 4.80g, glycine 6.20g, alanine 8.50g, proline 7.10g, asparagine 0.55g, glutamic acid 5.70g, serine 3.70g, N-acetyl equivalent to tyrosine 0.70g -L-tyrosine), and vitamin B 6 10g, vitamin B 1 3mg, vitamin B 2 3mg, vitamin B 3 40mg, pantothenic acid 8mg, biotin 0.4mg, folic acid 0.6mg, vitamin B 12 10 μg; Vitamin C 4g.
[0058] Vitamins can be directly added to compound amino acid injection, or added to 5% glucose sodium chloride injection. If you are a diabetic, you can add vitamins to 0.9% sodium chloride injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com