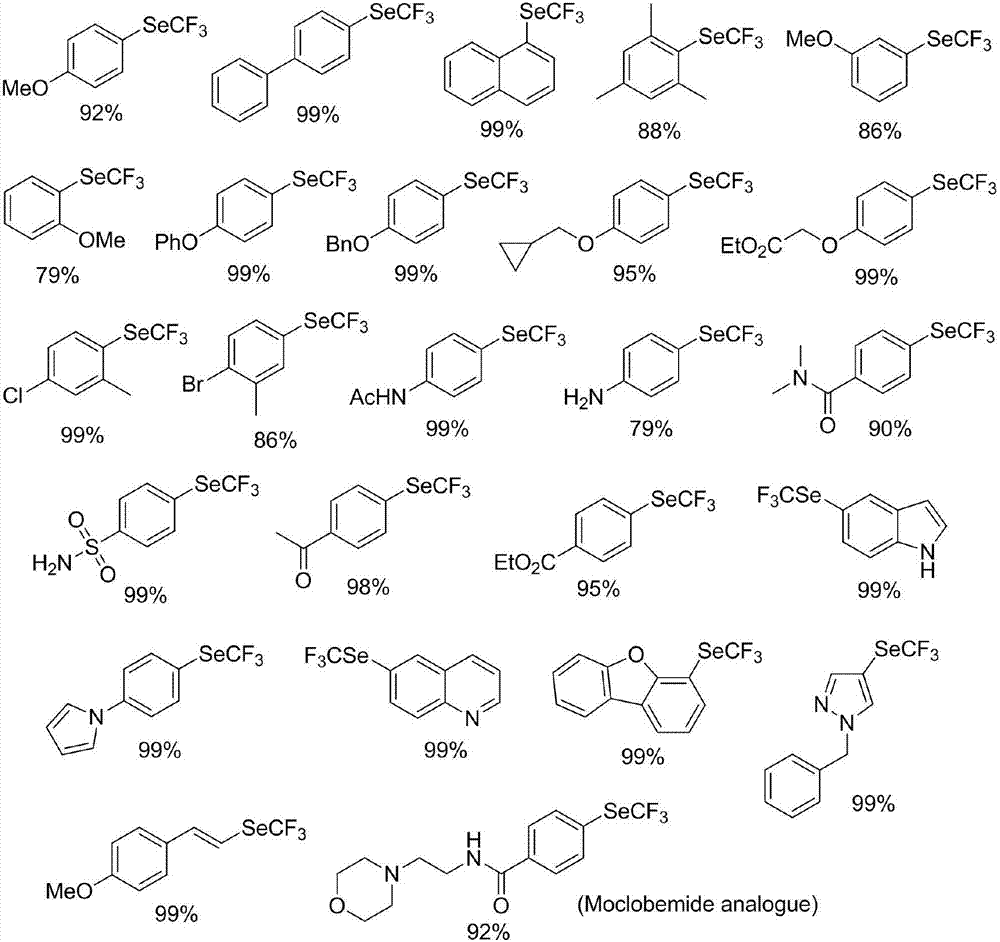
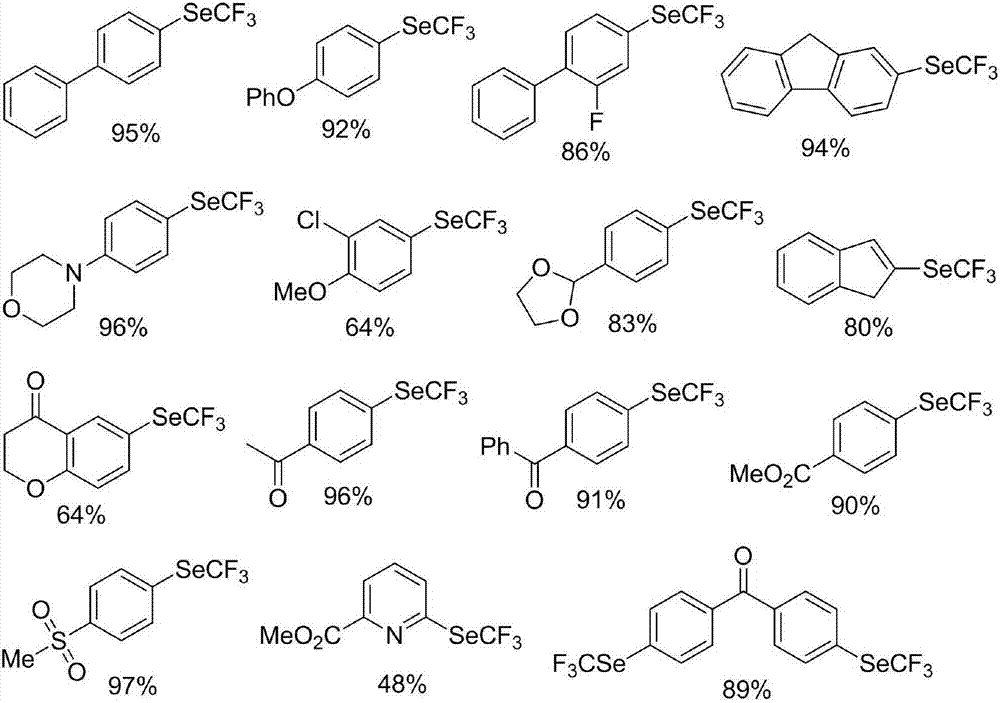
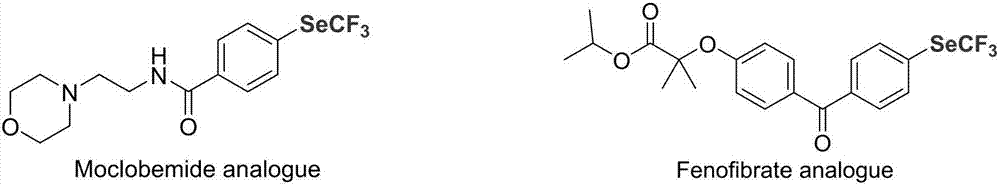Preparation method of nickel-catalyzed trifluoromethyl diaryliodonium
A technology of trifluoromethyl aryl and fluoromethyl aryl, which is applied in the field of preparation of trifluoromethyl aryl selenide, can solve the problems of narrow application range of substrates, difficult acquisition of catalysts, poor atom economy, etc., and achieves The effect of wide application range of substrates, high yield, and low requirements for equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] At room temperature, in a 100mL nitrogen-protected reaction flask, add 4-iodoanisole (4.68g, 20mmol), tetramethylammoniumtrifluoromethylselenide (5.33g, 24mmol), 2,2'-bipyridine (0.31 g, 2mmol) and bis-(1,5-cyclooctadiene)nickel (0.28g, 1mmol), then add tetrahydrofuran (50mL) with a syringe, and react at room temperature for 2 hours. The reaction solution was spin-dried, and the residue was subjected to silica gel column chromatography (n-hexane eluent) to obtain 4-trifluoromethylselenoanisole (4.69 g, 92%).
[0043] 1 H NMR (400MHz, CDCl 3 )δ7.66(d, J=8.8Hz, 2H), 6.91(d, J=8.8Hz, 2H), 3.83(s, 3H); 19 F NMR (376MHz, CDCl 3 )δ-37.2(s,3F); 13 C NMR (100MHz, CDCl 3 )δ161.4, 138.9, 122.5 (q, J = 333.0Hz), 115.2, 112.9, 55.3.
Embodiment 2
[0045]
[0046] At room temperature, in a 100mL nitrogen-protected reaction flask, add 4-iodoacetophenone (4.92g, 20mmol), tetramethylammoniumtrifluoromethylselenide (5.33g, 24mmol), 2,2'-bipyridine (0.31 g, 2mmol) and tetrakis(triphenylphosphine)nickel (1.11g, 1mmol), then tetrahydrofuran (50mL) was added by syringe, and reacted at room temperature for 2 hours. The reaction solution was spin-dried, and the residue was subjected to silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 20:1 (v / v)) to obtain 4-trifluoromethylselenoacetophenone (3.47g , 65%).
[0047] 1 H NMR (400MHz, CDCl 3 )δ7.95(d, J=8.3Hz, 2H), 7.83(d, J=8.2Hz, 2H), 2.62(s, 3H); 19 F NMR (376MHz, CDCl 3 )δ-35.2(s,3F); 13 C NMR (100MHz, CDCl 3 )δ197.2, 138.2, 136.6, 129.1, 128.2, 122.3 (q, J = 332.9Hz), 26.6.
Embodiment 3
[0049]
[0050] At minus 80 degrees Celsius, in a 100mL nitrogen-protected reaction flask, add ethyl 4-iodobenzoate (5.52g, 20mmol), tetramethylammoniumtrifluoromethylselenide (5.33g, 24mmol), 2,2'- Pyridine (0.31g, 2mmol) and bis-(1,5-cyclooctadiene)nickel (0.28g, 1mmol), and tetrahydrofuran (50mL) were added by syringe, and reacted at 0°C for 2 hours. The reaction solution was spin-dried, and the residue was subjected to silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 20:1 (v / v)) to obtain ethyl 4-trifluoromethylselenobenzoate (4.16 g, 70%).
[0051] 1 H NMR (400MHz, CDCl 3 )δ8.04(d, J=8.4Hz, 2H), 7.80(d, J=8.2Hz, 2H), 4.39(q, J=7.0Hz, 2H), 1.40(t, J=7.1Hz, 3H) ; 19 F NMR (376MHz, CDCl 3 )δ-35.3(s,3F); 13 C NMR (126MHz, CDCl 3 )δ165.7, 136.4, 132.2, 130.4, 127.9, 122.4 (q, J=332.4Hz), 61.4, 14.2. 1447,1396,1369,1302,1284,1273,1179,1130,1098,1066,1015,964,853,784,759,739,690cm -1 .HRMS-EIcalcd.for C 10 h 9 f 3 o 2 74 Se: 291.9779; F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com