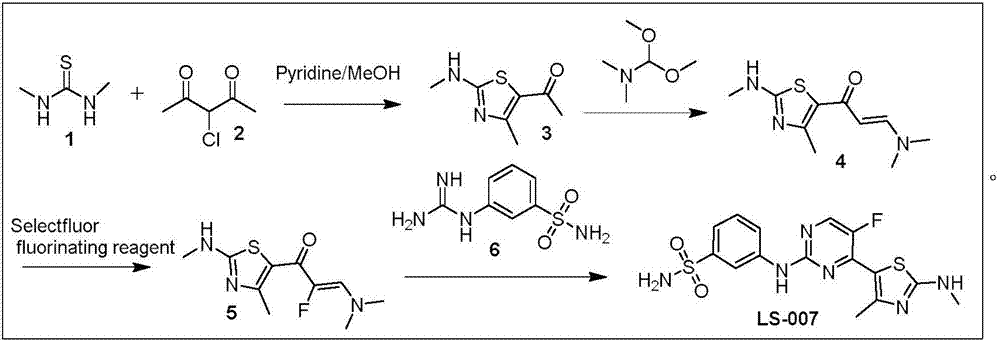
Preparation method of CDK (Cyclin-dependent Kinase) inhibitor
A technology of inhibitors and catalysts, applied in the field of pharmaceutical and chemical industries, can solve the problems of poor fluorination reaction, high environmental protection pressure, and waste liquid, etc., and achieve the effects of simple and feasible post-treatment, mild reaction conditions, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
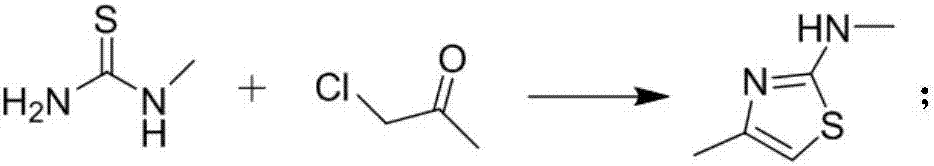
[0046] Embodiment 1 N, the preparation of 4-dimethylthiazol-2-amine
[0047] Add 9.01g (100.0mmol) of monomethylthiourea and 9.25g (100.0mmol) of 1-chloroacetone into the reaction flask, add an appropriate amount of methanol (100mL), lower the temperature to 0-30°C, and slowly add 7.91g (100.0mL) of pyridine dropwise. mmol) in methanol solution (20mL), after the dropwise addition, slowly raise the temperature to 20-40°C, continue to stir for 1-24 hours, then drop to -10-0°C, and stir at this temperature for 1-2h, filter, and use cold water to remove the solid washed with ethanol and dried under reduced pressure to obtain 10.3 g of white solid with a yield of 80.5%. 1 H NMR (CDCl 3 -d 3 ,400MHz), 4.6(br,1H), 5.7(s,1H), 2.6(s,3H), 2.1(s,3H); MS(EI)m / e(M+)129.0.
Embodiment 2
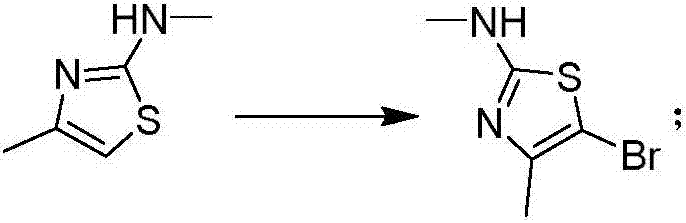
[0048] Example 2 Preparation of 5-bromo-N,4-dimethylthiazol-2-amine
[0049] Dissolve 9.0 g (70.0 mmol) of N,4-dimethylthiazol-2-amine in dichloromethane (150 mL), control the temperature at -5 to 5° C., and add 12.5 g (70.0 mmol) of NBS in batches. Keep it at -5~5°C, continue to react at -5~5°C for 0.5-2h after the addition is complete, spin the solvent to dry under reduced pressure, add ethanol (50mL), beat at room temperature for 1-2 hours, filter, and use the solid Ethanol wash. The solid was dried under reduced pressure to obtain 10.7 g (50.2 mmol) of 5-bromo-N,4-dimethylthiazol-2-amine with a yield of 73.8%. 1 H NMR (CDCl 3 -d 3 ,400MHz), 4.9(br,1H), 2.7(s,3H), 2.2(s,3H); MS(EI)m / e(M+)207.0.
Embodiment 3
[0050] The preparation of embodiment 3 key intermediate A
[0051] Under the protection of nitrogen, 2.07g (10.0mmol) of 5-bromo-N,4-dimethylthiazol-2-amine was added to the reaction flask, and then DMSO (15mL), 2.94g (30.0mmol) of potassium acetate, and bis (Pinacolate) diboron 2.55g (10.1mmol) and dppf palladium dichloride 0.22g (0.3mmol) catalyst, then replace the air in the bottle, heat to 65-100°C, and react at this temperature for 3-12 hours , after the reaction is complete, cool down, add water, extract three times with ethyl acetate, combine the extracts, wash with saturated saline solution, then dry with anhydrous magnesium sulfate, spin dry the filtrate under reduced pressure, and then purify with ethanol , filtered, the solid was washed with cold ethanol, and the solid was dried under reduced pressure to obtain 1.89 g of the key intermediate A with a yield of 70.5%. 1 H NMR (CDCl 3 -d 3 ,400MHz), 3.2(s,6H), 2.3(s,3H), 1.3(s,12H); MS(EI)m / e(M+)255.1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com