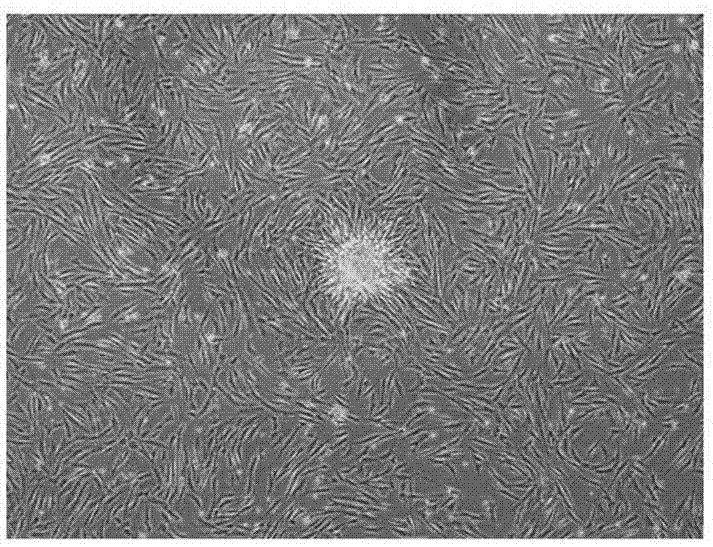
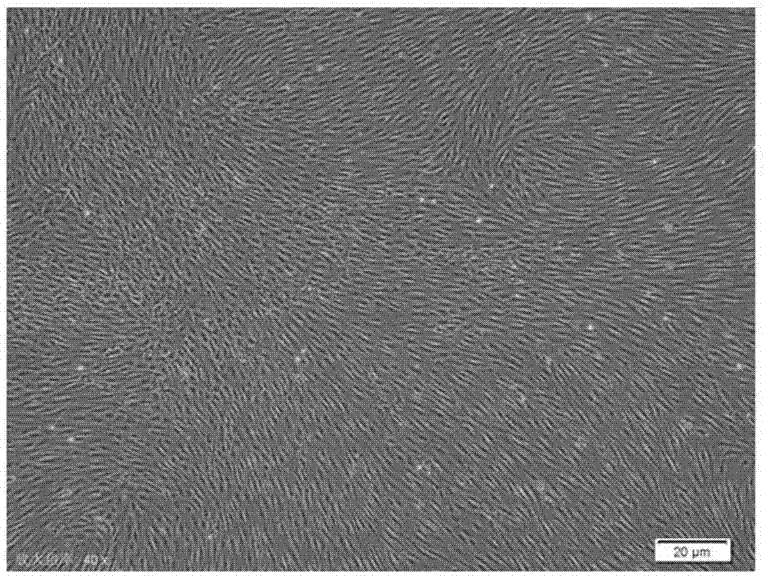
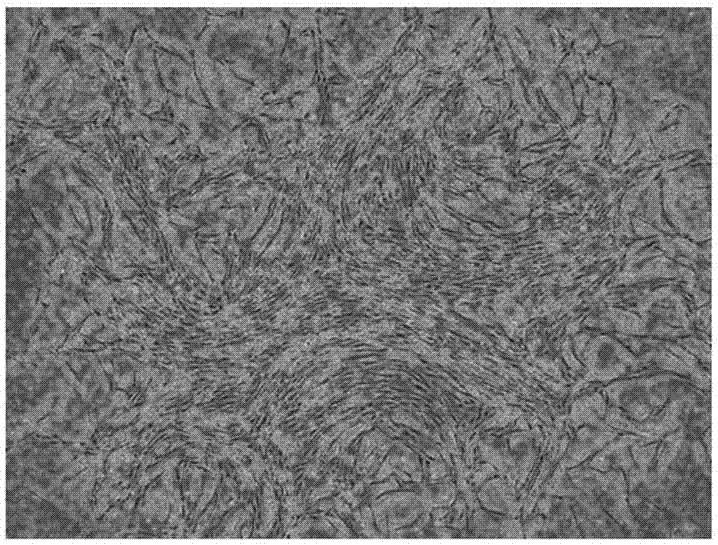
Culture medium for amplifying human mesenchymal stem cell and amplification method of culture medium
A technology of high-quality stem cells and medium, applied in the field of stem cell culture, to achieve the effects of reducing expansion time, avoiding differentiation, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Isolation, purification and culture of human bone marrow-derived mesenchymal stem cells
[0055] 1. Materials and reagents
[0056] Lymphocyte separation medium (Ficoll) (Tianjin Haoyang Biotechnology Co., Ltd.); culture medium DMEM (Hyclone Company), human AB serum (central blood station); cell counting kit (CCK-8) (Japan Dojindo Company); death kit (Dojindo Japan); epidermal growth factor (EGF), platelet-derived growth factor (PDGF), dexamethasone (DEX), penicillin streptomycin (Pen / strep), insulin iron selenide transfer protein (ITS), Human serum albumin (HSA) was produced by Peprotech; fluorescently labeled mouse anti-human PE-CD29, FITC-CD34, PE-CD44, FITC-CD45 antibodies (eBioscience, USA); FC500 flow cytometer and corresponding software ( American Beckman Coulter Company).
[0057] 2. Culture medium components
[0058] 1) Stem cell culture medium
[0059] Calculated by 1 liter, the dosage of each component is as follows:
[0060] MCDB 50ml, insulin transferr...
Embodiment 2
[0069] Isolation and culture of human umbilical cord human mesenchymal stem cells
[0070] 1. Materials and reagents
[0071] Same as Example 1
[0072] 2. Culture medium components
[0073] 1) Stem cell culture medium
[0074] Calculated by 1 liter, the dosage of each component is as follows:
[0075] MCDB 50ml, insulin transferrin (ITS) 200μl, human serum albumin (HSA) 60μl, penicillin streptomycin (Pen / strep) 1ml, dexamethasone (DEX) 10μl, add DMEM medium to 1 liter.
[0076] 2) Expansion medium
[0077] Calculated by 1 liter, the dosage of each component is as follows:
[0078] MCDB 50ml, insulin transferrin (ITS) 200μl, human serum albumin (HSA) 60μl, penicillin streptomycin (Pen / strep) 1ml, dexamethasone (DEX) 10μl, linoleic acid (LA) 6ml, human Source AB type serum 5ml, platelet-derived growth factor (PDGF) 50μl, epidermal growth factor (EGF) 60μl, add DMEM medium to 1 liter.
[0079] 3. Culture method (adhesion method)
[0080] Including the following steps:
...
Embodiment 3
[0089] Isolation and culture of human umbilical cord human mesenchymal stem cells
[0090] 1. Materials and reagents
[0091] Same as Example 1
[0092] 2. Culture medium components
[0093] 1) Stem cell culture medium
[0094] Calculated by 1 liter, the dosage of each component is as follows:
[0095] MCDB 50ml, insulin transferrin (ITS) 200μl, human serum albumin (HSA) 60μl, penicillin streptomycin (Pen / strep) 1ml, dexamethasone (DEX) 10μl, add DMEM medium to 1 liter.
[0096] 2) Expansion medium
[0097] Calculated by 1 liter, the dosage of each component is as follows:
[0098] MCDB 50ml, insulin transferrin (ITS) 200μl, human serum albumin (HSA) 60μl, penicillin streptomycin (Pen / strep) 1ml, dexamethasone (DEX) 10μl, linoleic acid (LA) 6ml, human Source AB type serum 5ml, platelet-derived growth factor (PDGF) 50μl, epidermal growth factor (EGF) 60μl, add DMEM medium to 1 liter.
[0099] 3. Culture method (enzyme digestion method)
[0100] Including the following s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com