A kind of heterogeneous gibel crucian carp anti-CYHV-2 oral recombinant spore vaccine and its preparation method
A heterogeneous gibel crucian carp, cyhv-2 technology, applied in recombinant DNA technology, antiviral agents, pharmaceutical formulations, etc., can solve problems such as safety risks and loss of immune induction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
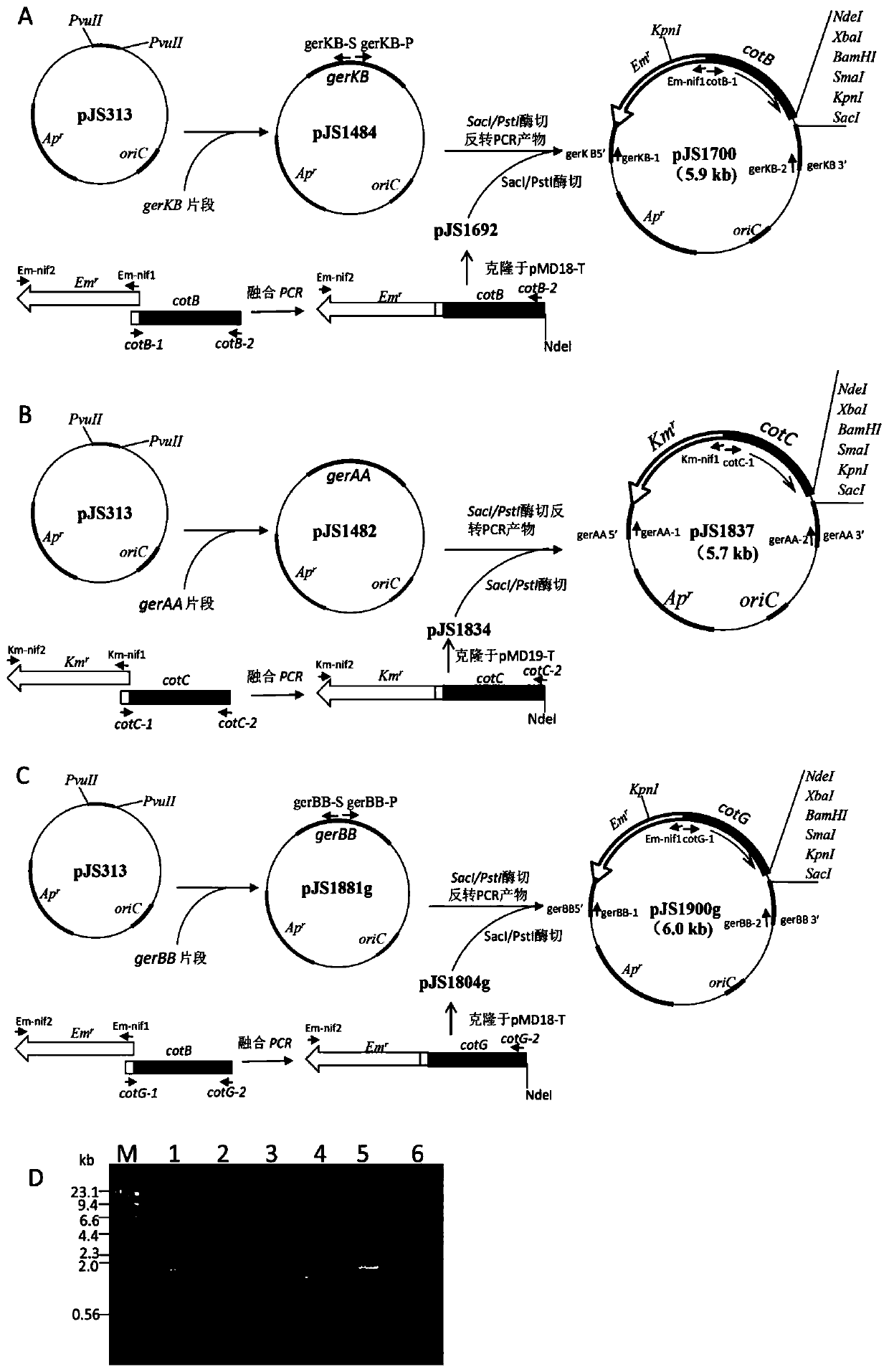
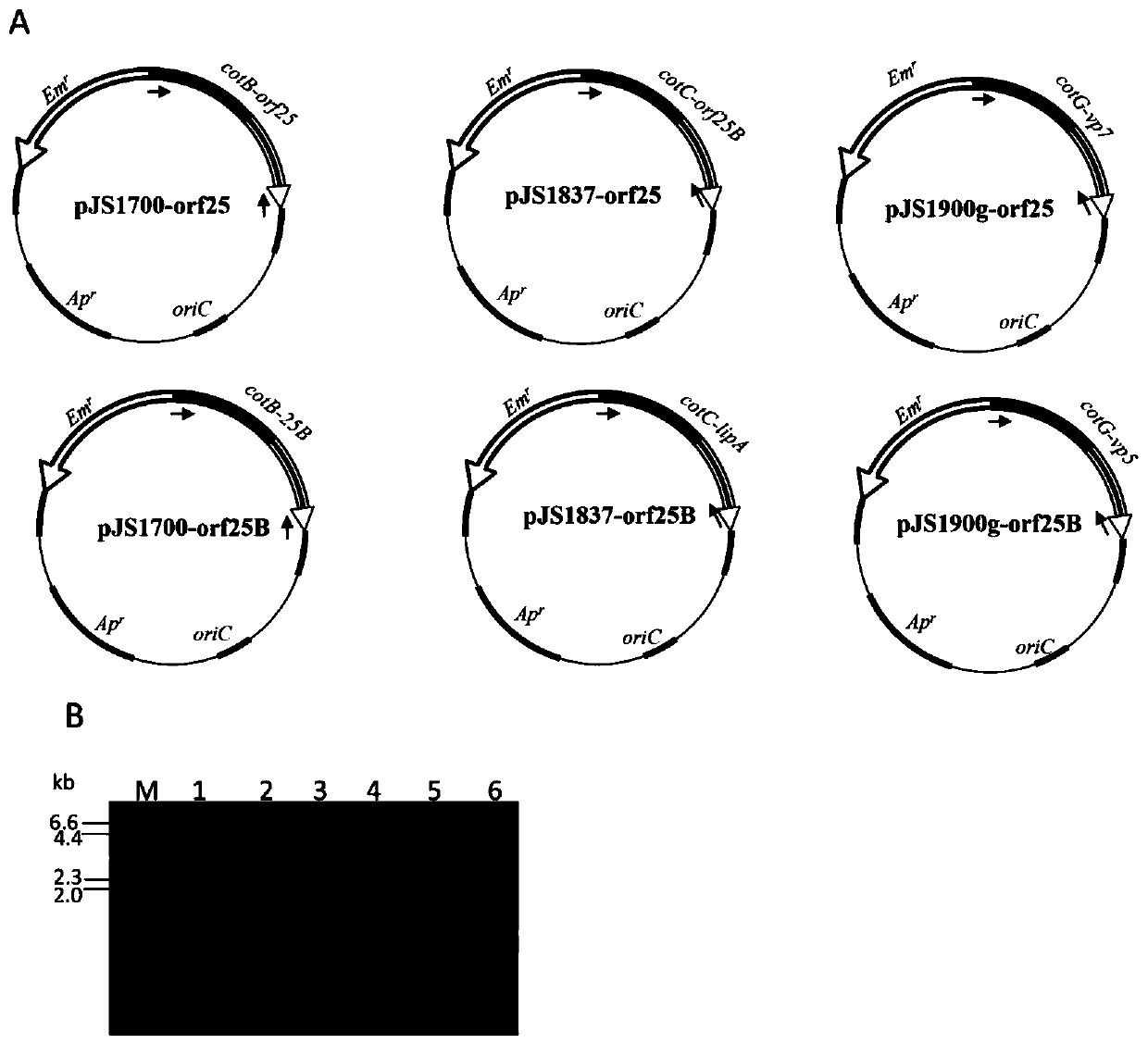
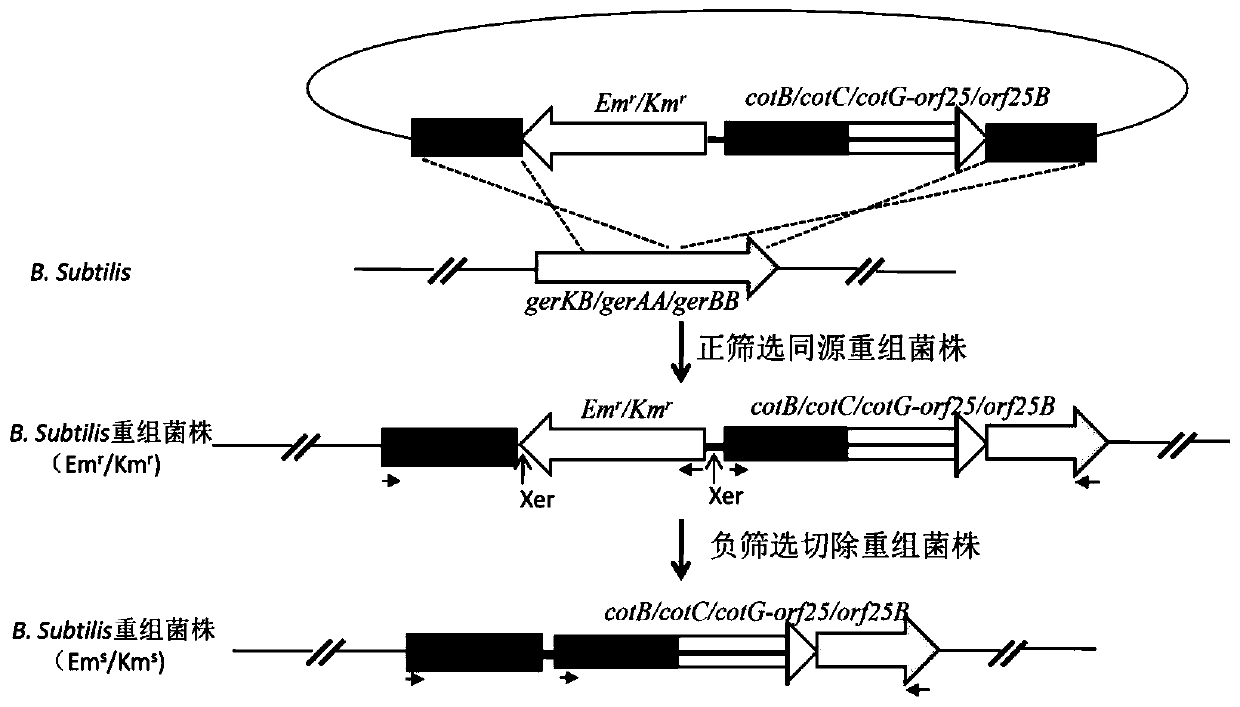
[0054] Preparation of Oral Recombinant Spore Vaccine Displaying CyHV-2 Antigen ORF25 Using gerKB as Integration Site
[0055] 1. Molecular Biology Operations
[0056] 1.1 Extraction of Bacillus subtilis chromosome
[0057] Collect 10mL B.subtilis 168(trp - ) culture, add 0.5mL TE to suspend the pellet. Add 30 μL lysozyme (100 mg / mL) to each microcentrifuge tube, and react at 37°C for 1 h; add 50 μL 10% sodium dodecylsulfonate (SDS) and 20 μl 20 mg / mL proteinase K, shake evenly, and React at ℃ for 2 hours, add an equal volume of phenol and chloroform to extract, take the supernatant, add 2 times the volume of ethanol, and after 2 hours at room temperature, centrifuge at 12,000g for 10 minutes, discard the supernatant, wash the DNA precipitate with 500 μL of 75% ethanol, and Remove inorganic salt ions. After the DNA precipitate is dried, add 30-50 μL TE or ddH 2 O dissolved the DNA and stored it at -20°C for later use.
[0058] 1.2 Molecular biology technical operation
...
Embodiment 2
[0096] Preparation of Oral Recombinant Spore Vaccine Displaying CyHV-2 Antigen ORF25 Using gerAA as Integration Site
[0097] 1. Molecular biology manipulation and bacterial transformation
[0098] The operation method described in 1. and 2. in Example 1 is the same.
[0099] 2. Construction of an integrative vector with gerAA as the integration site
[0100] 2.1 Construction of an integrative platform vector with gerAA as the integration site
[0101] Using gerA-1 and gerA-2 as primers, B. subtilis 168 chromosome as a template, PCR amplified gerAA gene fragment, T4 DNA polymerase filled in and cloned into pUC18 plasmid pJS313 (NdeI restriction site was destroyed, Molecular Cloning : Experiment Manual "Second Edition, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, 1989) at the PvuII site, the resulting recombinant plasmid is pJS1482. PstI and SacI restriction sites exist in the gerA fragment of pJS1482 plasmid. Km-nif1 and Km-nif2 primers containing nif seque...
Embodiment 3
[0113] Recombinant Bacillus subtilis spores displaying ORF25 with gerBB as integration site without antibiotic resistance gene marker
[0114] 1. Molecular biology manipulation and bacterial transformation
[0115] Same as the method described in 1. and 2. in Example 1.
[0116] 2. Construction of an integrative vector with gerBB as the integration site
[0117] 2.1 Construction of an integrative platform vector with gerBB as the integration site
[0118] Using gerBB-1 and gerBB-2 as primers, B. subtilis 168 chromosome as a template, PCR amplified gerBB gene fragment, T4 DNA polymerase filled in and cloned into pUC18 plasmid pJS313 (NdeI restriction site was destroyed, Molecular Cloning : Experiment Manual "Second Edition, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, 1989) at the PvuII site, the resulting recombinant plasmid is pJS1881g. Using gerBB-P and gerBB-S as primers and pJS1881g as a template, the inverse PCR amplification product was digested with P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com