A method for acid hydrolysis of calcium arsenate and/or calcium arsenite
A technology of calcium arsenite and calcium arsenate, applied in the field of waste treatment, can solve problems such as secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0042] In the present invention, as another embodiment, the step (1) is preferably specifically:
[0043] adding calcium arsenate and / or calcium arsenite to sulfuric acid solution for metathesis reaction, adding sulfuric acid solution during the reaction to maintain the pH value of the reaction at less than or equal to 5.0;
[0044] The pH value of the sulfuric acid solution is less than or equal to 5.0.
[0045] In the present invention, calcium arsenate and / or calcium arsenite are added into sulfuric acid solution to perform metathesis reaction, and sulfuric acid solution is added during the reaction to maintain the pH value of the reaction at less than or equal to 5.0. In the present invention, the pH value of the sulfuric acid solution is less than or equal to 5.0, preferably 0.5-4.
[0046] In the present invention, calcium arsenate and / or calcium arsenite is preferably mixed with water, prepared into a slurry and then added to the sulfuric acid solution, and the content o...
Embodiment 1
[0058] Using lime to reduce arsenic-containing wastewater (H 2 SO 4 : 25g / L; As(III): 6g / L) adjusted to pH 2.0, solid-liquid separation to obtain gypsum, continue to add lime to the filtrate to pH 11.0, solid-liquid separation, to obtain a mixture containing calcium arsenite.
[0059] Add 30mL of deionized water to 30g of the mixture containing calcium arsenite, stir until a slurry is formed, slowly add the slurry dropwise into 150ml of nitric acid solution using a peristaltic pump under mechanical stirring, add nitric acid to dissolve the calcium arsenite and stabilize the pH to 1.6, and sodium sulfate was added dropwise to the nitric acid solution, and the pH of the reaction system was maintained at 3.0. After the dropwise addition was completed, the stirring was continued for 0.5 hours, and the solid and liquid were separated to obtain calcium sulfate precipitate and arsenic-containing solution.
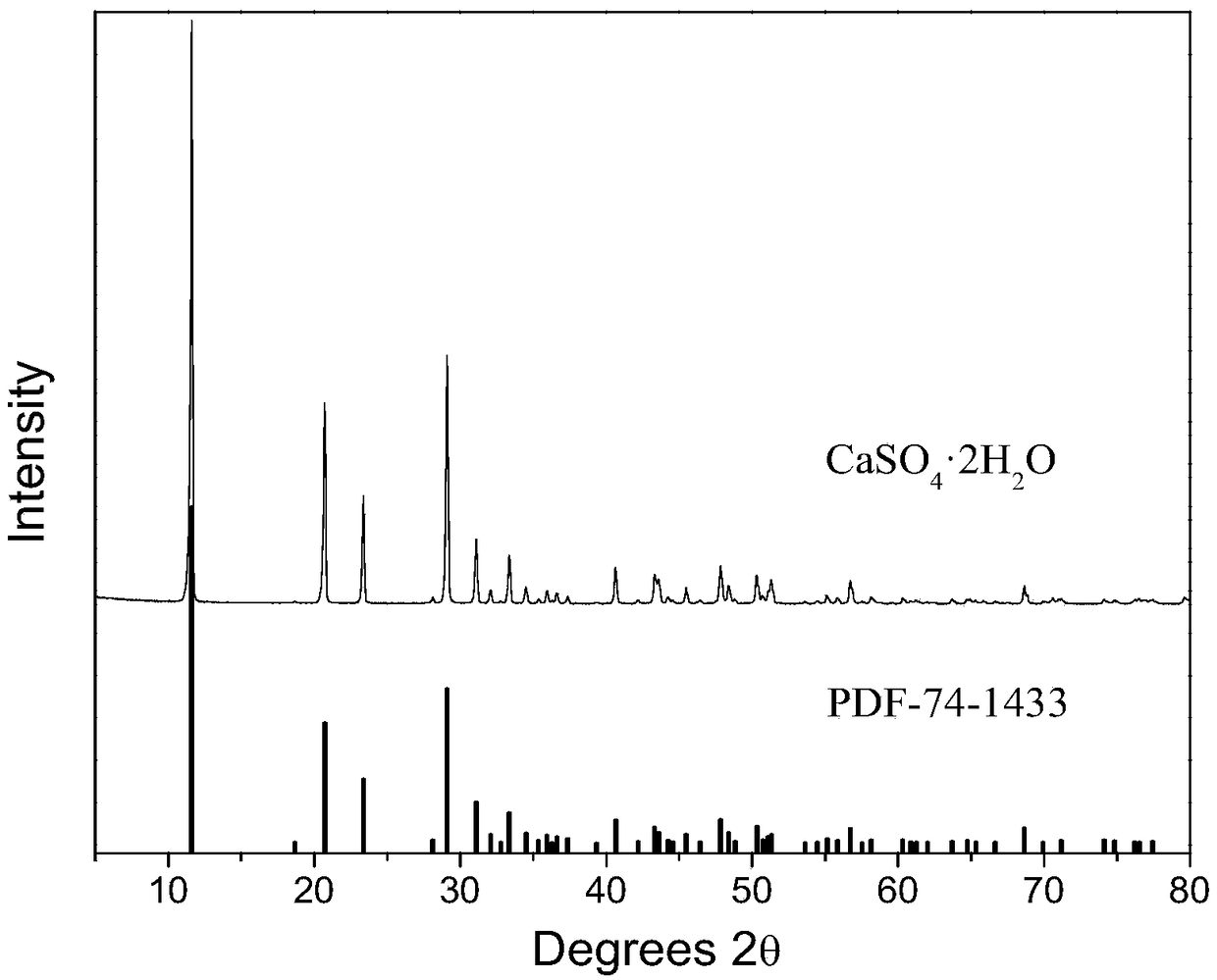
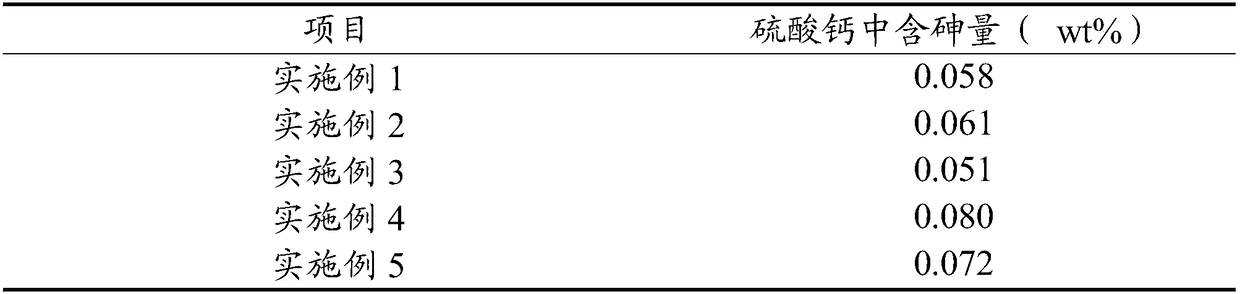
[0060] Gained calcium sulfate is analyzed using X-ray diffraction pattern, a...
Embodiment 2
[0063] Using lime to reduce arsenic-containing wastewater (H 2 SO 4 : 10g / L; As (V): 2g / L) adjusted to pH 2.0, solid-liquid separation to obtain gypsum, continue to add lime to the filtrate to pH 11.0, solid-liquid separation, to obtain a mixture containing calcium arsenate.
[0064] Add 30ml of deionized water to 30g of calcium arsenate-containing mixture, stir until a slurry is formed, slowly add the slurry dropwise to 150ml of sulfuric acid solution with pH 1.6 under mechanical stirring, and add dropwise to the sulfuric acid solution Sulfuric acid solution to maintain the pH of the reaction system at 2.6, continue stirring for 0.5 hours after the dropwise addition, and separate solid and liquid to obtain calcium sulfate precipitate and arsenic-containing solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com