Meningococcal polysaccharide conjugate vaccine soluble microneedle patch and preparation method thereof
A combined vaccine and soluble technology, applied in the field of transdermal drug delivery, can solve the problems of inaccurate dosing and uncontrollable drug release rate, and maximize drug utilization efficiency, controllable drug release rate, and simple and convenient use. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 microneedle mold
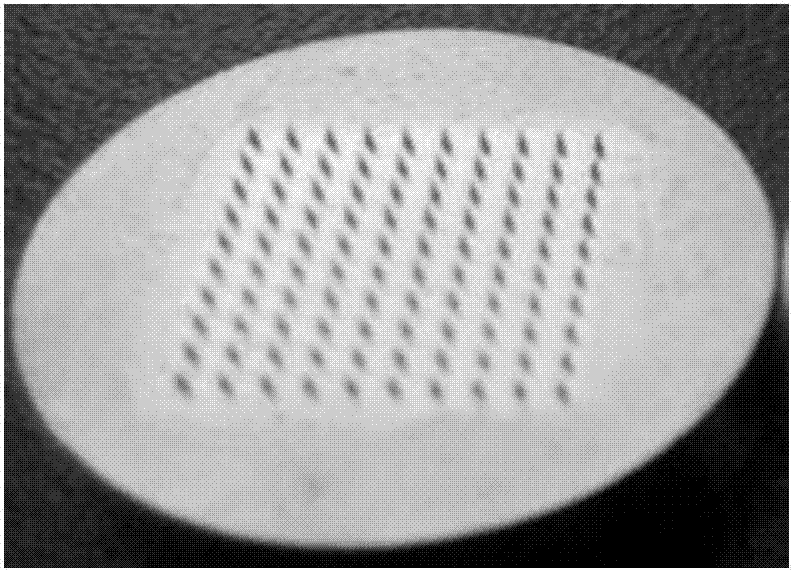
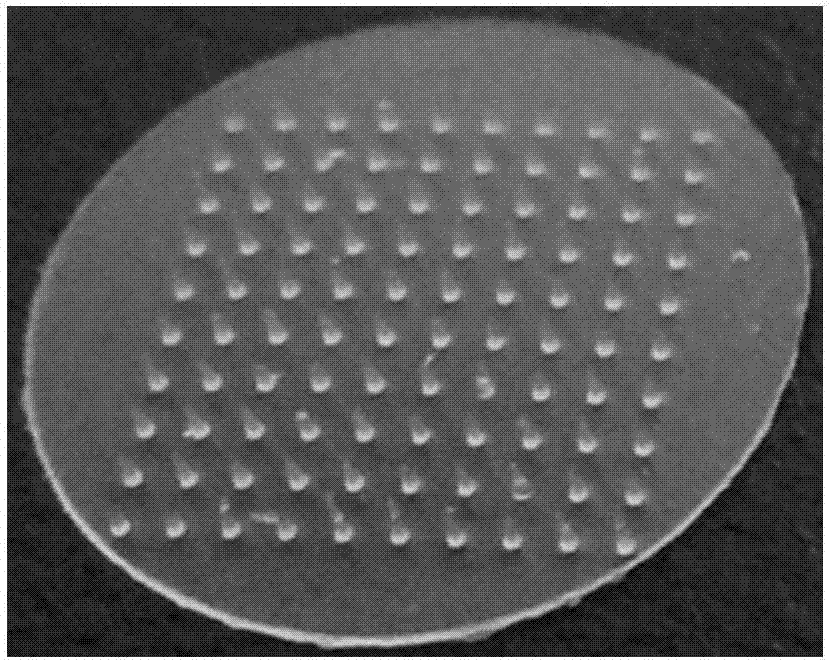
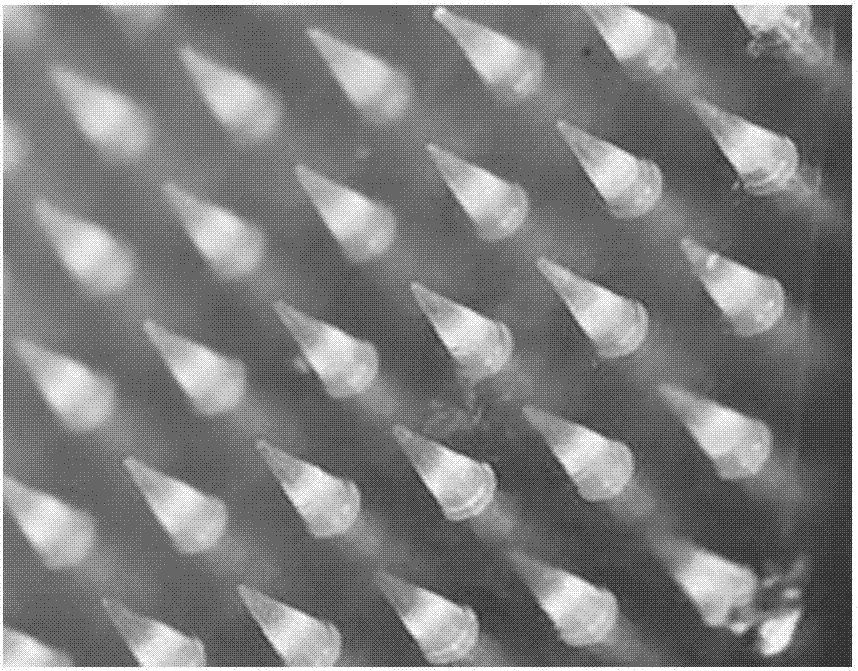
[0055] (1) Preparation of the male mold: use computer-aided design (CAD) to design the shape, height, spacing, and density of the microneedle arrays of the male mold. The specific specifications of the mold are: the height of the microneedles is 800 μm, and the pitch 900μm, needle bottom diameter 300μm, array 10×10 rows, 100 tapered microneedles, microneedle array size 1cm×1cm, the number of microneedle arrays can be adaptively designed according to the size of the mold. Transform design drawings into digital information of digital machine tools through digital-to-analog conversion, and select brass and stainless steel as processing materials to process and shape according to the design size; figure 1 shown.
[0056] (2) Preparation of the female mold: Place the prepared brass and stainless steel male molds in absolute ethanol, ultrasonically clean the organic cooling liquid remaining on the surface during the preparat...
Embodiment 2
[0058] In this example, the effect of different contents of the A+C meningococcal meningitis polysaccharide conjugate vaccine on the mechanical properties of the microneedles was investigated.
[0059] (1) Preparation of different doses of meningococcal polysaccharide-conjugated vaccine microneedle needle tip liquid: take A+C group meningococcal polysaccharide-conjugated vaccine and dilute it with deionized water to be 50mg / ml, 40mg / ml, 30mg / ml, 20mg / ml respectively , 15mg / ml, 10mg / ml, 5mg / ml of seven different concentrations of A+C meningococcal meningococcal polysaccharide conjugated vaccine, draw 2.0ml of the vaccine solution of the above six concentrations respectively, add sodium hyaluronate 0.55g, dextran 40 (DEX40) 0.40g, povidone K30 (PVP K30) 0.05g, vortex and mix well, after fully swelling, let stand overnight to dissolve, and then get soluble microneedle tip liquid (sodium hyaluronate: dextran 40: povidone The mass ratio of K30:water is 11:8:1:40). The added sodium...
Embodiment 3
[0087] In this example, through the comparative study of two different administration methods of soluble microneedle transdermal immunization and injection immunization, the difference in immune effect between the soluble microneedle transdermal immunization method and the subcutaneous injection immunization method was evaluated. At the same time, the influence of soluble microacupuncture with different needle heights on the immune effect is investigated, which mainly includes the following steps:
[0088] In this embodiment, the molecular weight of sodium hyaluronate is 7775, the molecular weight of dextran 40 is 37000, and the molecular weight of povidone K30 is 40000.
[0089] (1) Preparation of a control group injected with meningitis polysaccharide conjugated vaccine:
[0090] Group A+C meningococcal polysaccharide conjugated vaccine injection control group (CI1): take 5 bottles each of group A meningococcal polysaccharide conjugated vaccine freeze-dried powder and group ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com