Boactivity peptide gene therapy method realized by propeptide recombination
A bioactive peptide and gene therapy technology, applied in gene therapy, peptide preparation methods, chemical instruments and methods, etc., can solve problems such as high cost, failure to meet industrial production requirements, and inability to realize the prevention and treatment of bioactive peptides. To achieve the effect of ensuring identification and effective cutting and preventing degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Construction of recombinant propeptide of anti-tumor angiogenesis peptide Anginex and its gene therapy
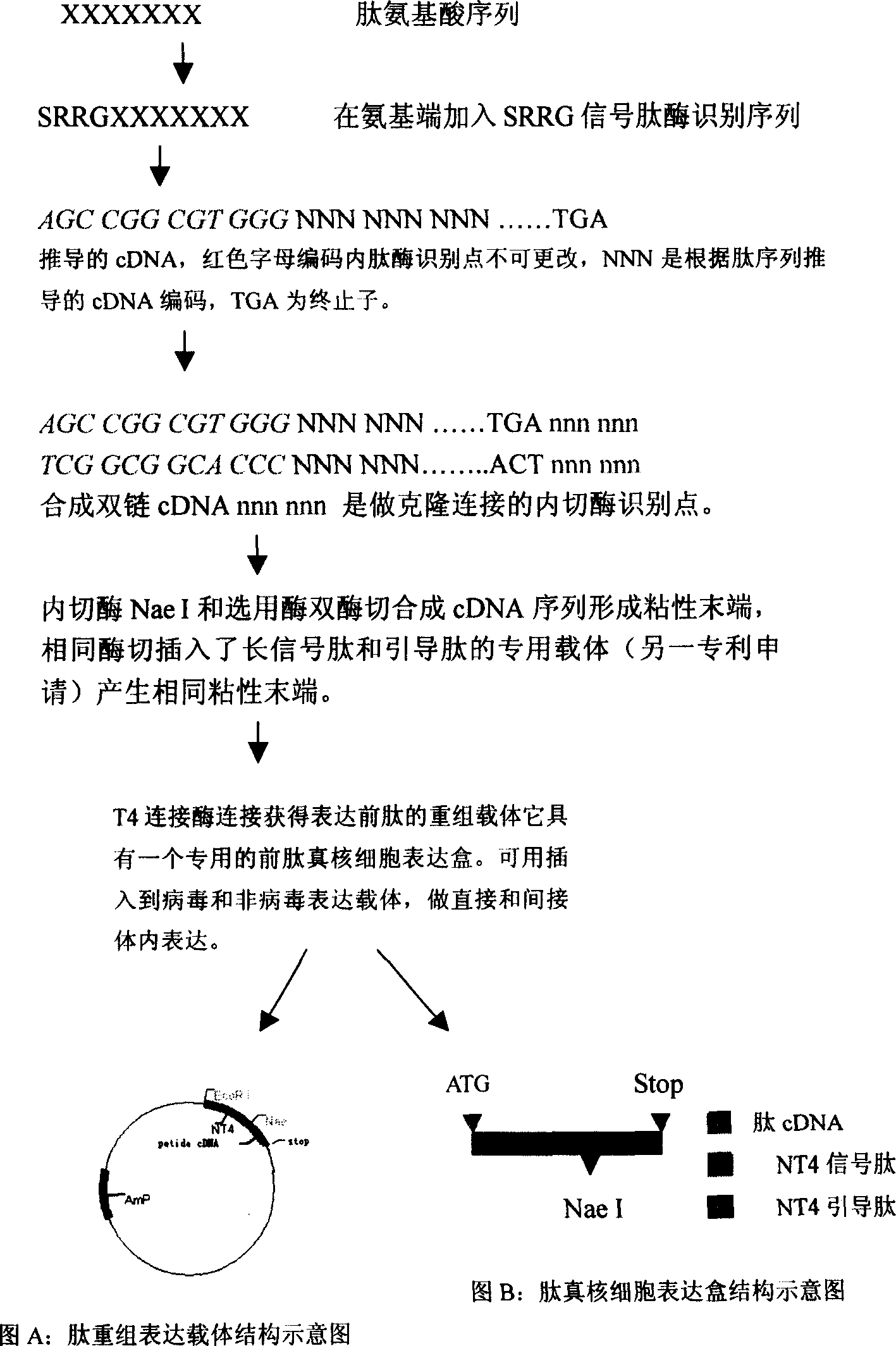
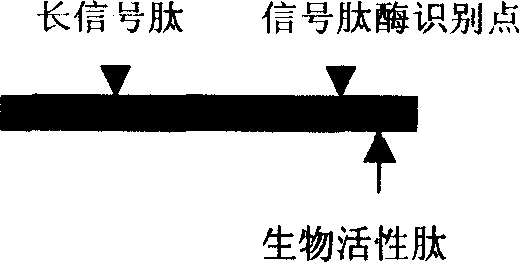
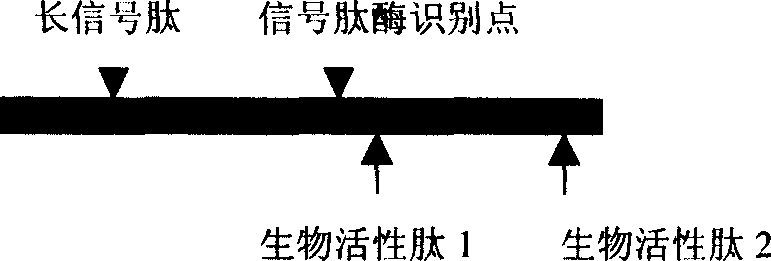
[0031] (1) Determine the sequence of the bioactive peptide: XXXXXX in the figure represents the amino acid sequence of the selected bioactive peptide, and each X represents an amino acid residue. According to the desired biological effect, the user selects a peptide with specific biological activity from the published literature or through his own preliminary research findings, such as the anti-tumor effect of promoting apoptosis and inhibiting tumor angiogenesis Peptides, antiviral infection peptides that antagonize HIV or HCV invading target cell receptors, aptamer peptides that block intracellular signal transduction, and nutritional peptides that activate cell functions and treat senile degenerative diseases, etc. The mechanisms and principles of action of these bioactive peptides are diverse, and the subcellular locations where they act are also diver...
Embodiment 2
[0128] Example 2, construction of TAT-VHLβ structural propeptide and anti-kidney cancer effect:
[0129] The first step: determine the sequence of biologically active peptides: Renal cell carcinoma is a common malignant tumor, recurrence and metastasis after surgery are common, and the tumor is not sensitive to radiation and chemical anticancer drug treatment, so the patient's prognosis Difference. Recent biological studies of renal cancer cells have revealed that the proliferation and metastasis of the cells depend on insulin-like growth factor 1 (insulin-like growth factor 1, ILGF1) receptor signaling. Yon Hippel-Lindau (VHL) is a tumor suppressor gene, and its expression product inhibits the ILGF1 receptor signal through the combination with protein kinase Cδ, leading to the apoptosis of renal cancer cells, and has the effect of treating renal cancer. However, there are technical difficulties in using VHL protein to treat renal cancer solid tumors. One is that protein mole...
Embodiment 3
[0178] Example 3, construction of recombinant adeno-associated virus expressing anti-Alzheimer's Humanin propeptide and its protective effect on neurons.
[0179] Alzheimer's disease (AD) is a common degenerative disease of the central nervous system in the elderly. The etiology is currently unknown, but the pathology is characterized by neuronal loss, senile plaque formation, and neurofibrillary tangles. A 24-amino acid residue polypeptide-Humanin was discovered using molecular biology screening techniques, which can antagonize AD pathological changes caused by various known genetic causes, especially neural apoptosis. We used this patented technology to construct a recombinant propeptide expressing Humanin, expressed the propeptide using an adeno-associated virus vector, transduced rat cortical neurons, and successfully prevented amyloid peptide (the most important neuron-promoting apoptosis in AD) Apoptosis induced by dead substances).
[0180] Step 1: Determine the seque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com