Triazole structure unit-based tetradentate cyclometalated platinum (II) and palladium (II) complex phosphorescent materials
A phosphorescent luminescent material, metal platinum technology, applied in luminescent materials, palladium organic compounds, platinum group organic compounds, etc., can solve problems such as spectral blue shift, and achieve the effect of reducing energy, strong molecular rigidity, and high phosphorescent luminescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
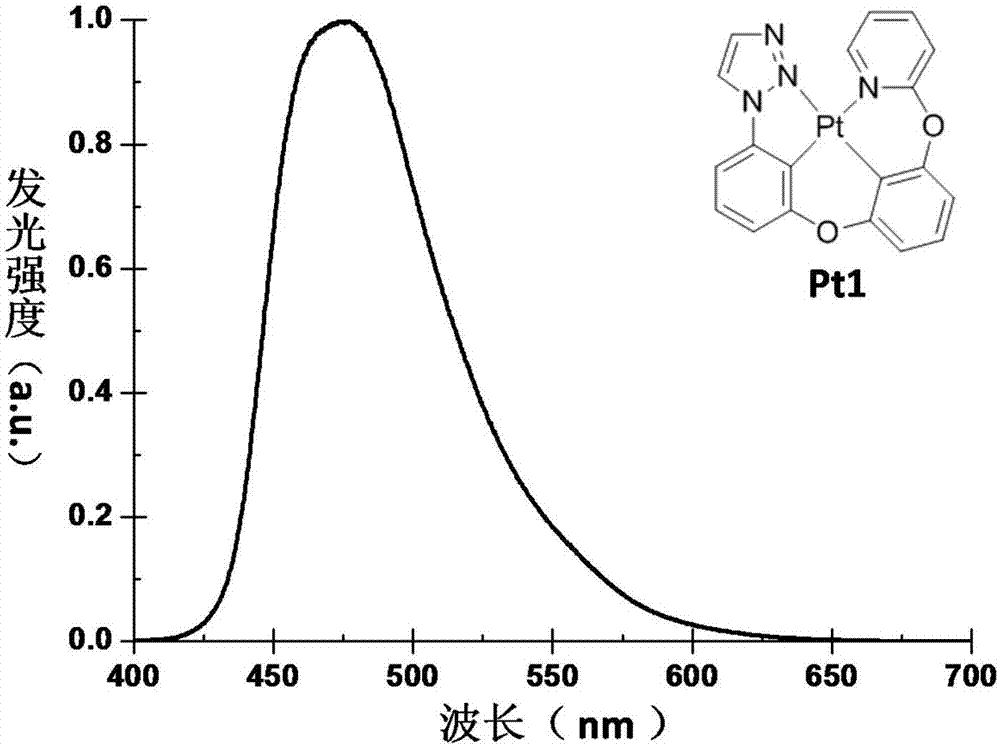
[0044] Example 1: The synthetic route of the phosphorescent material Pt1 of tetracyclic metal platinum complex is as follows:
[0045]
[0046] Synthesis of 3-(3-(1-(1H-1,2,3-triazolyl))phenoxy)-(2-pyridyloxy)benzene Ligand 1: Reaction to a dry 25 mL with a magnetic rotor 1-(3-hydroxyphenyl)-1H-1,2,3-triazole (644.6mg, 4.0mmol, 1.0eq), 3-bromo-(2-pyridyloxy)benzene (1.20g , 4.8mmol, 1.2eq), cuprous iodide (76.2mg, 0.4mmol, 0.1eq), ligand 2-pyridinecarboxylic acid (98.5mg, 0.8mmol, 0.2eq), potassium phosphate (1.78g, 8.4mmol, 2.1 eq). The nitrogen was purged three times, then the solvent dimethyl sulfoxide (5 mL) was added. Then the reaction mixture was stirred at 105°C for 3 days, cooled to room temperature, diluted with a large amount of ethyl acetate, filtered and washed with ethyl acetate. The obtained filtrate was washed 3 times with water and dried over anhydrous sodium sulfate. Filtration, the filtrate was rotary evaporated under reduced pressure to remove the sol...
Embodiment 2
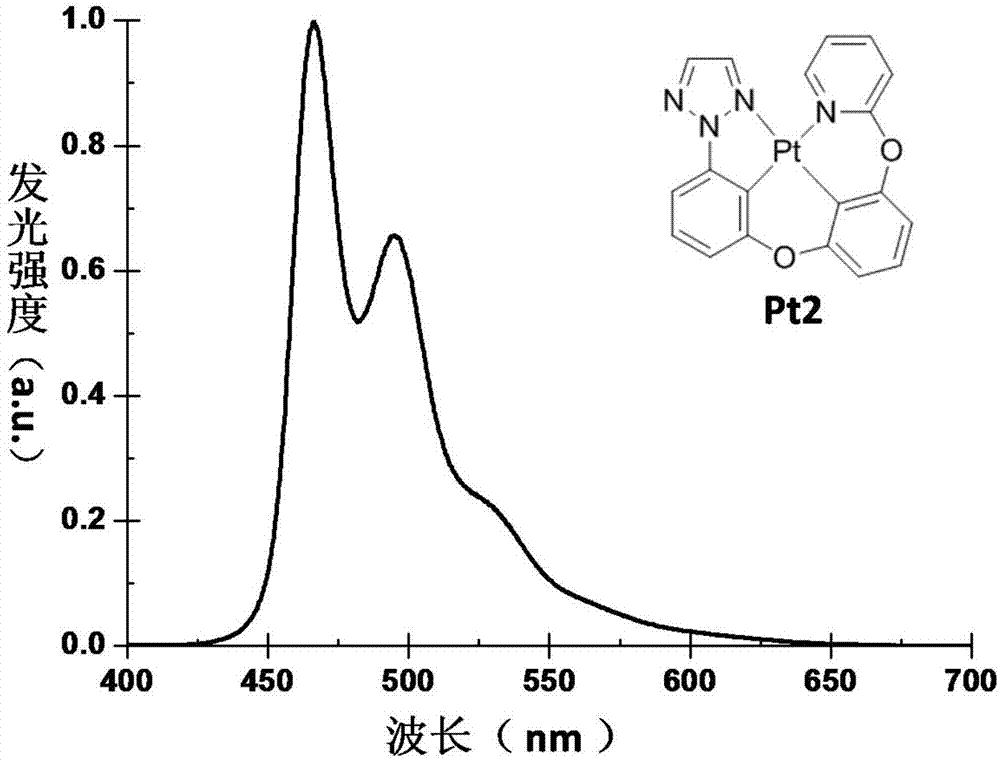
[0049] Embodiment 2: The synthesis route of tetradentate ring metal platinum complex phosphorescent material Pt2 is as follows:
[0050]
[0051] Synthesis of 3-(3-(2-(2H-1,2,3-triazolyl))phenoxy)-(2-pyridyloxy)benzene ligand 2: reaction to a dry 25 mL with a magnetic rotor 2-(3-hydroxyphenyl)-2H-1,2,3-triazole (644.6mg, 4.0mmol, 1.0eq), 3-bromo-(2-pyridyloxy)benzene (1.20g , 4.8mmol, 1.2eq), cuprous iodide (76.2mg, 0.4mmol, 0.1eq), ligand 2-pyridinecarboxylic acid (98.5mg, 0.8mmol, 0.2eq), potassium phosphate (1.78g, 8.4mmol, 2.1 eq). The nitrogen was purged three times, then the solvent dimethyl sulfoxide (5 mL) was added. Then the reaction mixture was stirred at 105°C for 3 days, cooled to room temperature, diluted with a large amount of ethyl acetate, filtered and washed with ethyl acetate. The obtained filtrate was washed 3 times with water and dried over anhydrous sodium sulfate. Filtration, the filtrate was rotary evaporated under reduced pressure to remove the s...
Embodiment 3
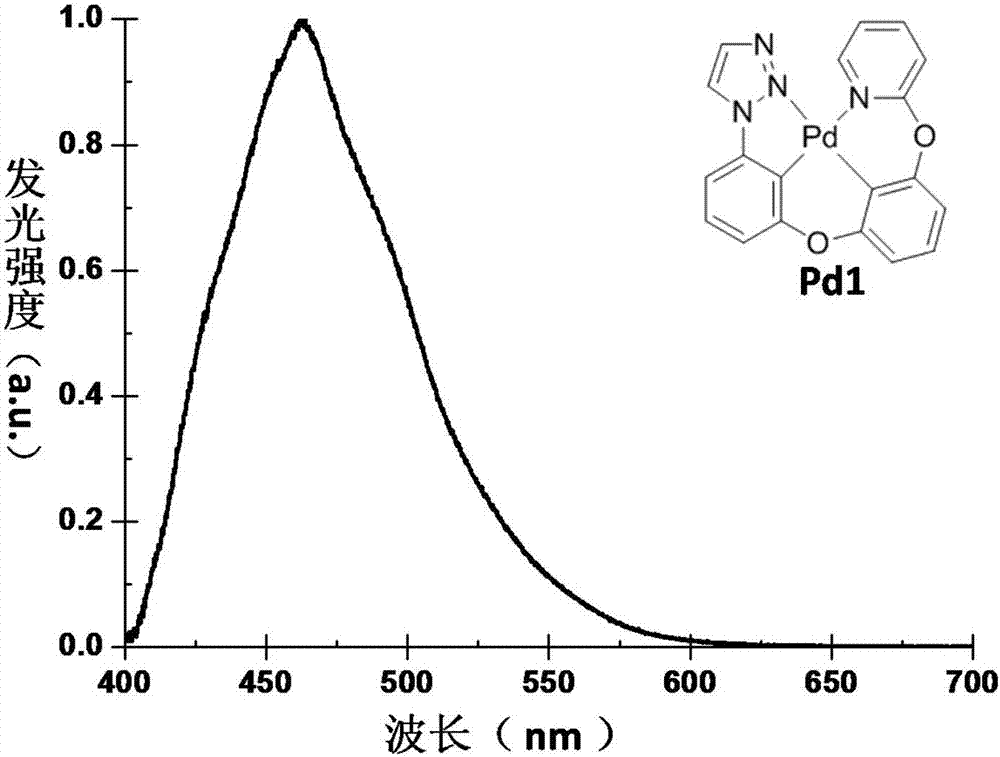
[0054] Example 3: The synthesis route of the phosphorescent material Pd1 of tetradentate ring metal platinum complex is as follows:
[0055]
[0056] Add Ligand 1 (486.8mg, 1.5mmol, 1.0eq) obtained in the previous step, Pd(OAc) 2 (364.2mg, 1.6mmol, 1.1eq) and n Bu 4 NBr (48.3 mg, 0.15 mmol, 0.1 eq). The nitrogen was purged three times, then the solvent acetic acid (88 mL) was added. The reaction was then stirred at 110°C for 2 days. The reaction mixture was cooled to room temperature, and the solvent was removed by rotary evaporation under reduced pressure. The obtained crude product was separated and purified by silica gel column chromatography, eluting agent (petroleum ether / dichloromethane=1:1-1:4), and 417.9 mg of white solid was obtained. rate of 64%.
[0057] 1 H NMR (500MHz, DMSO-d 6 ):δ6.94(dd, J=6.0,1.5Hz,1H),7.04(dd,J=8.0,1.0Hz,1H),7.11(dd,J=8.0,0.5Hz,1H),7.23(t, J=8.0Hz, 1H), 7.36(t, J=8.0Hz, 1H), 7.44-7.48(m, 2H), 7.68(dd, J=8.0, 1.0Hz, 1H), 8.17(ddd, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com