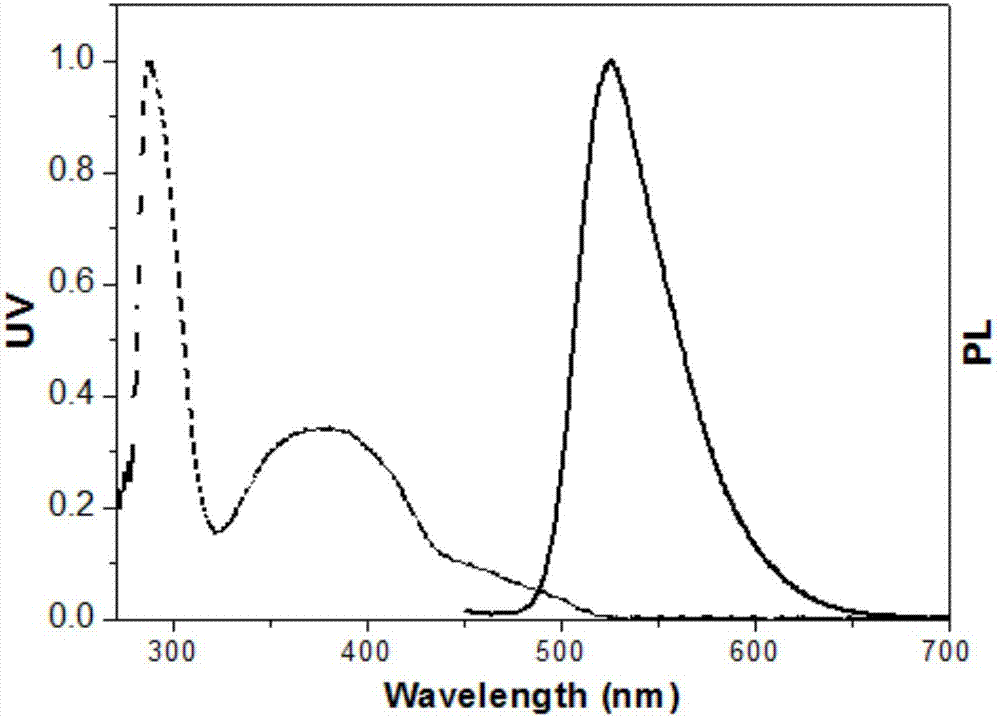
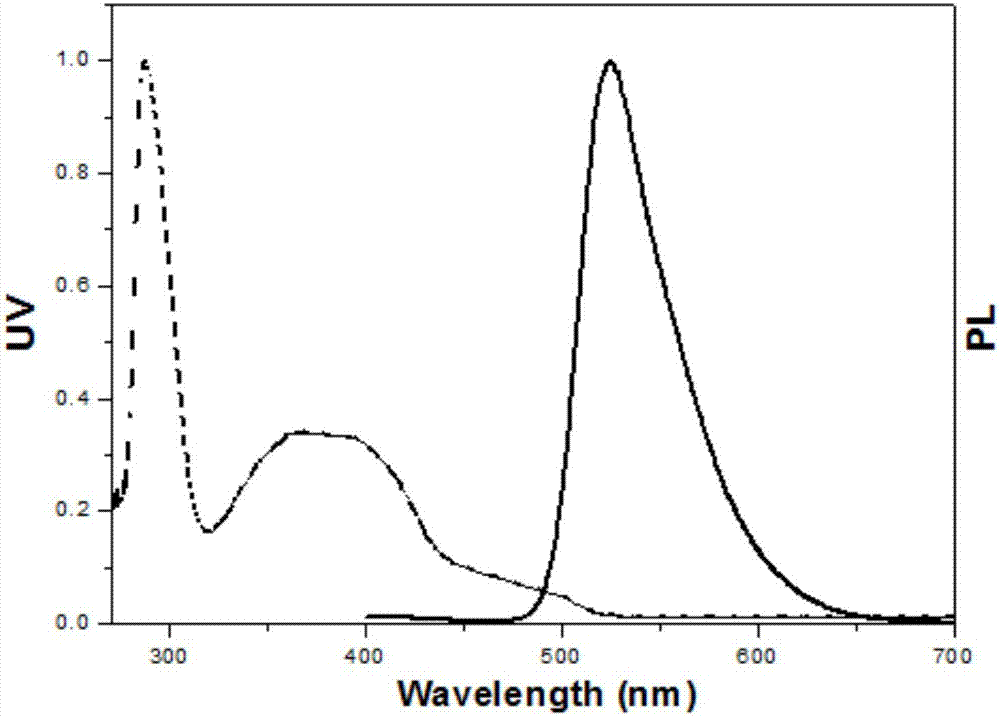
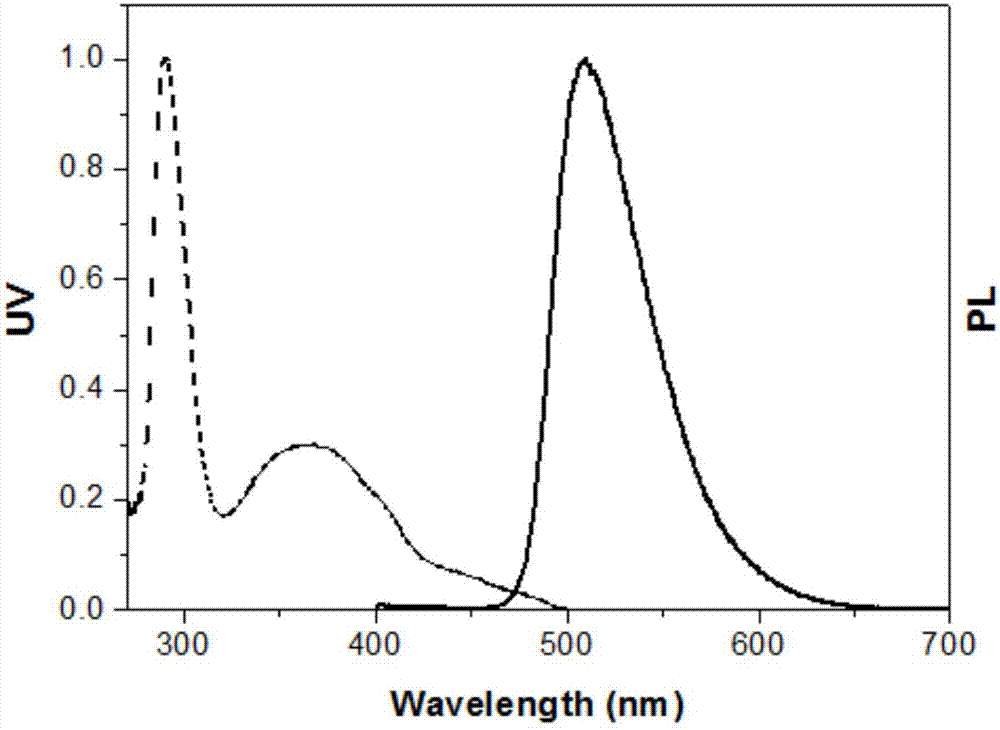
Pyridazine iridium complexes containing alkyl steric-hindrance groups, and preparation method and application thereof
An iridium complex and pyridazine technology, applied in the field of pyridazine iridium complex and its preparation, can solve the problems of weak iridium complex luminescence concentration, obvious quenching, expensive raw materials or raw material adaptability, etc. Luminescence quenching, small phosphorescence quenching, and the effect of reducing intermolecular forces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add 2.3g (10mmol) 1,4-dichloro-5,6,7,8-tetrahydro-5,8-ethanephthalazine, 2.4g (20mmol) phenylboronic acid and 1.4g (10mmol) potassium carbonate to 250mL In the round bottom flask, add 0.23g (0.2mmol) Pd (PPh 3 ) 4 , then poured into 30mL tetrahydrofuran and 15mL water, N 2 Protection, reflux at 100°C for 12h. Cool, spin dry THF, extract with dichloromethane to obtain the lower organic phase, spin dry, and separate by silica gel column chromatography with petroleum ether: ethyl acetate = 1:1 eluent to obtain 0.62 g of 1,4-diphenyl - 5,6,7,8-Tetrahydro-5,8-ethanephthalazine as a white solid in 20% yield. The NMR spectrum of the product is: 1 H NMR (400MHz, CDCl 3 )δ7.71(dd,J=7.5,5.8Hz,5H),7.54-7.48(m,5H),3.44(s,2H),1.88(d,J=8.0Hz,4H),1.46(d,J =7.6Hz, 4H). The mass spectrum of product is: MS ((+)-ESI): m / z=313.33 (calcd.313.17forC 22 h 21 N 2 ,[M+H + ]).
[0045] 0.94g (3mmol) 1,4-diphenyl-5,6,7,8-tetrahydro-5,8-ethanephthalazine, 0.56g (1mmol) IrCl 3 .(tht) ...
Embodiment 2
[0048]Add 2.3g (10mmol) 1,4-dichloro-5,6,7,8-tetrahydro-5,8-ethanephthalazine, 4.8g (40mmol) phenylboronic acid and 8.4g (60mmol) potassium carbonate to 500mL In the round bottom flask, add 1.15g (1mmol) Pd (PPh 3 ) 4 , then poured into 60mL tetrahydrofuran and 30mL water, N 2 Protection, reflux at 150°C for 5h. Cool, spin dry tetrahydrofuran, extract with dichloromethane to obtain the lower organic phase, spin dry, and separate by silica gel column chromatography with petroleum ether: ethyl acetate = 1:1 eluent to obtain 0.78 g of 1,4-diphenyl - 5,6,7,8-Tetrahydro-5,8-ethanephthalazine as a white solid in 25% yield. The nuclear magnetic spectrum and mass spectrum of product are basically the same as in Example 1.
[0049] 1.57g (5mmol) 1,4-diphenyl-5,6,7,8-tetrahydro-5,8-ethanephthalazine, 0.56g (1mmol) IrCl 3 .(tht) 3 , 0.246g (3mmol) anhydrous sodium acetate and 60mL decahydronaphthalene solution, N 2 Protected and reacted at 250°C for 40h. Cool down after the react...
Embodiment 3
[0051] 2.3g (10mmol) 1,4-dichloro-5,6,7,8-tetrahydro-5,8-ethanephthalazine, 5.4g (20mmol) 4-(trifluoromethyl)phenylboronic acid pina Add alcohol ester and 1.4g (10mmol) potassium carbonate to a 250mL round bottom flask, add 0.2g (0.3mmol) PdCl 2 (dppf), then pour 30mL tetrahydrofuran and 15mL water, N 2 Protection, reflux at 100°C for 12h. Cool, spin dry tetrahydrofuran, extract with dichloromethane to obtain the lower organic phase, spin dry, and use petroleum ether: ethyl acetate = 1:1 eluent silica gel column chromatography to obtain 2.0 g 1,4-bis(p- Trifluoromethylphenyl)-5,6,7,8-tetrahydro-5,8-ethanephthalazine as a white solid, yield 44%. The NMR spectrum of the product is: 1 H NMR (400MHz, CDCl 3 ) δ 7.82 (s, 8H), 3.40 (s, 2H), 1.93 (d, J=8.0Hz, 4H), 1.47 (d, J=7.8Hz, 4H). 19 F NMR (CDCl 3 ,367MHz) δ-62.73(s, 6F). The mass spectrum of product is: MS ((+)-ESI): m / z=449.42 (calcd.449.14for C 24 h 19 f 6 N 2 ,[M+H + ]).
[0052] 1.35g (3mmol) 1,4-bis(p-trifluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com